Abstract
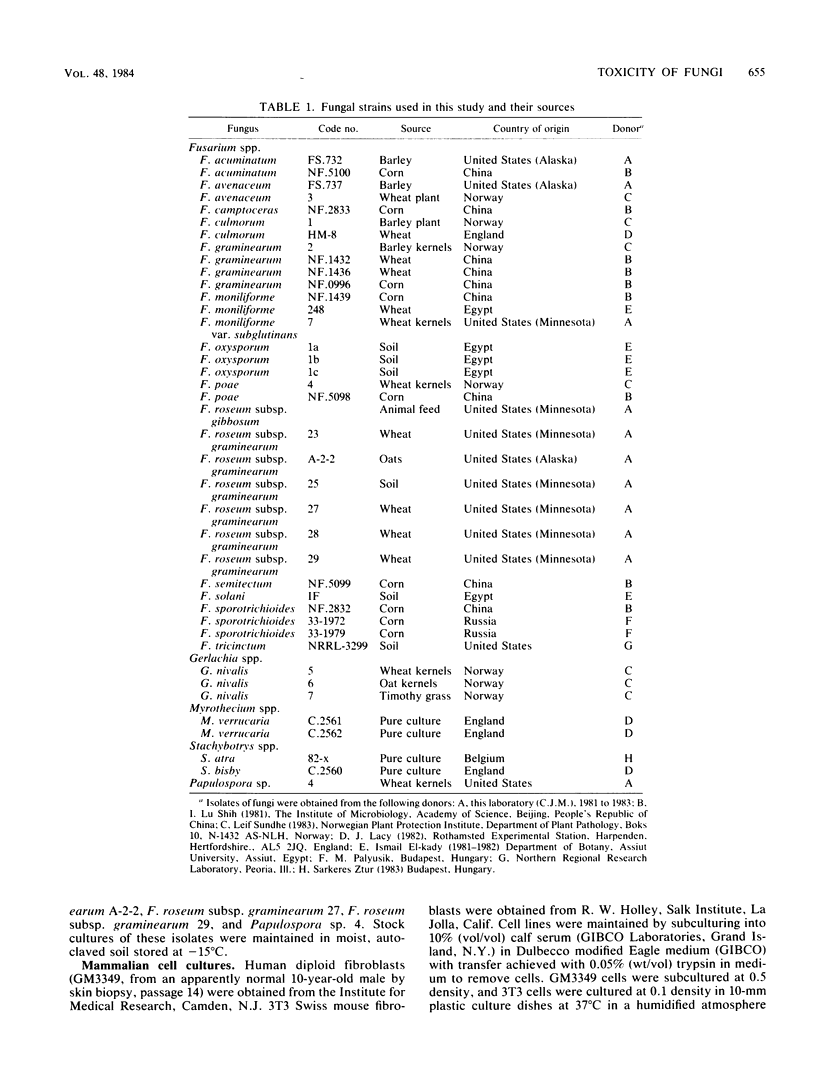
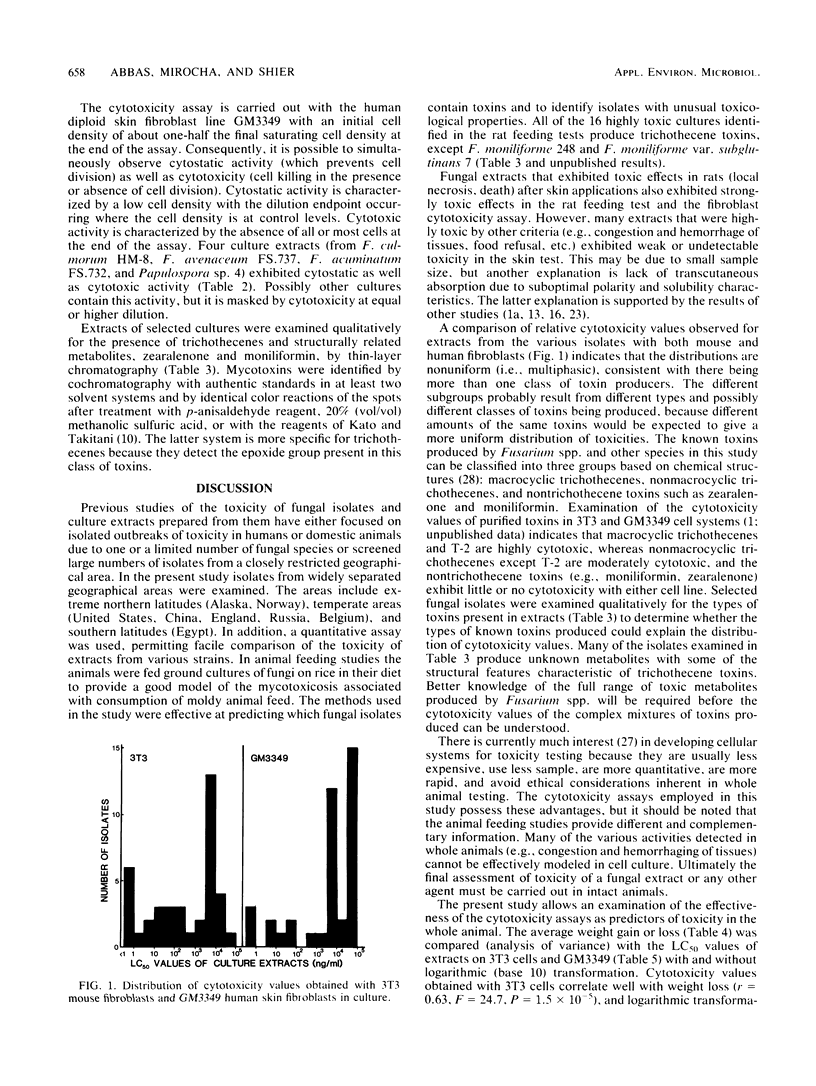
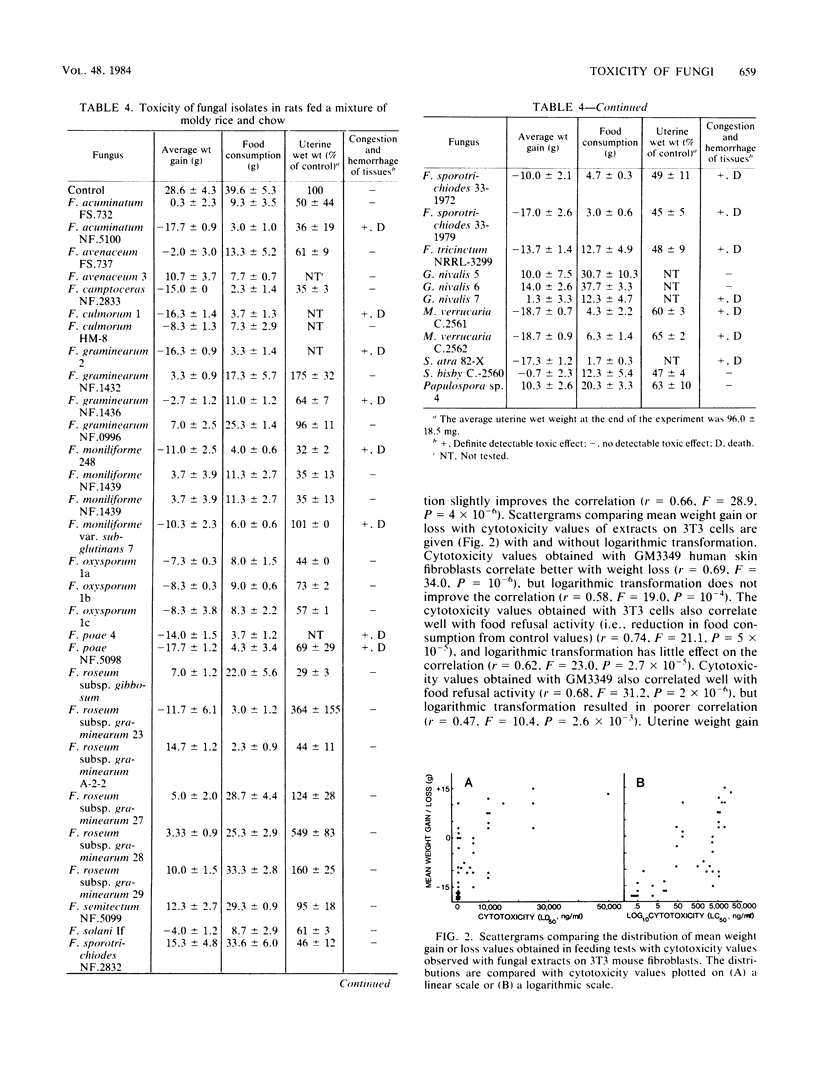
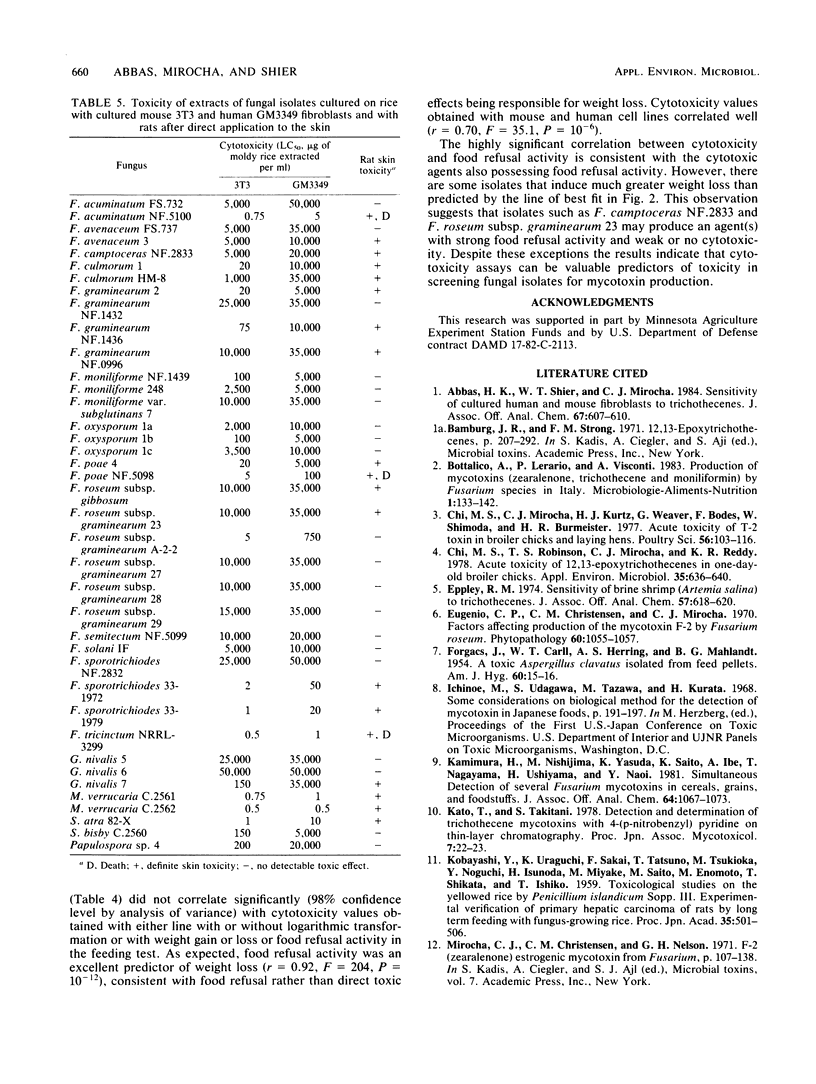
Thirty-nine isolates of fungi obtained from foodstuffs and soil samples from various parts of the world have been identified. The isolates were grown on a solid rice medium, and extracts were prepared with 50% aqueous methanol. The extracts were examined for toxicity in the following systems: (i) cytotoxicity to cultured normal human diploid skin fibroblasts (proliferating and nonproliferating) and mouse fibroblasts; (ii) skin toxicity after topical application on rats; and (iii) rat feeding tests in which rats were examined for death, overt pathological effects including congestion and hemorrhage of tissues, weight loss, food refusal, and uterine growth. Sixteen culture extracts were highly toxic as indicated by death, congestion and hemorrhage of tissues, and net weight loss. One half of the isolates were highly cytotoxic (50% lethal concentration, 0.01 to 5 micrograms/ml) as indicated by the ability to cause death and disintegration of 3T3 Swiss mouse fibroblasts and human diploid skin fibroblasts during 3 to 4 days in culture. The remainder were moderately cytotoxic (50% lethal concentration, 5 to 250 micrograms/ml). Four culture extracts were highly toxic by some clinical criteria but did not cause congestion and hemorrhage of tissues and were weakly cytotoxic (50% lethal concentration, 250 to 5,000 micrograms/ml). Six culture extracts exhibited moderate toxicity (weight loss only) and low cytotoxicity (50% lethal concentration, 3,000 to 50,000 micrograms/ml). Four culture extracts caused uterine enlargement as the major clinical sign, suggesting the presence of zearalenone. Eleven culture extracts were weakly cytotoxic and caused no major clinical signs, except skin toxicity in two extracts.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas H. K., Shier W. T., Mirocha C. J. Sensitivity of cultured human and mouse fibroblasts to trichothecenes. J Assoc Off Anal Chem. 1984 May-Jun;67(3):607–610. [PubMed] [Google Scholar]
- Chi M. S., Mirocha C. J., Kurtz H. J., Weaver G., Bates F., Shimoda W., Burmeister H. R. Acute toxicity of T-2 toxin in broiler chicks and laying hens. Poult Sci. 1977 Jan;56(1):103–116. doi: 10.3382/ps.0560103. [DOI] [PubMed] [Google Scholar]
- Chi M. S., Robison T. S., Mirocha C. J., Reddy K. R. Acute toxicity of 12,13-epoxytrichothecenes in one-day-old broiler chicks. Appl Environ Microbiol. 1978 Apr;35(4):636–640. doi: 10.1128/aem.35.4.636-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppley R. M. Sensitivity of brine shrimp (Artemia salina) to trichothecenes. J Assoc Off Anal Chem. 1974 May;57(3):618–620. [PubMed] [Google Scholar]
- Eugenio C. P., Christensen C. M., Mirocha C. J. Factors affecting production of the mycotoxin F-2 by Fusarium roseum. Phytopathology. 1970 Jul;(7):1055–1057. doi: 10.1094/phyto-60-1055. [DOI] [PubMed] [Google Scholar]
- FORGACS J., CARLL W. T., HERRING A. S., MAHLANDT B. G. A toxic Aspergillus clavatus isolated from feed pellets. Am J Hyg. 1954 Jul;60(1):15–26. doi: 10.1093/oxfordjournals.aje.a119700. [DOI] [PubMed] [Google Scholar]
- Kamimura H., Nishijima M., Yasuda K., Saito K., Ibe A., Nagayama T., Ushiyama H., Naoi Y. Simultaneous detection of several Fusarium mycotoxins in cereals, grains, and foodstuffs. J Assoc Off Anal Chem. 1981 Sep;64(5):1067–1073. [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Behrens J., Schauerhamer B. Uterotropic activity of cis and trans isomers of zearalenone and zearalenol. Appl Environ Microbiol. 1978 May;35(5):986–987. doi: 10.1128/aem.35.5.986-987.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathre S. V., Mirocha C. J., Christensen C. M., Behrens J. Monoacetoxyscirpenol. A new mycotoxin produced by Fusarium roseum Gibbosum. J Agric Food Chem. 1976 Jan-Feb;24(1):97–103. doi: 10.1021/jf60203a053. [DOI] [PubMed] [Google Scholar]
- Rabie C. J., Marasas W. F., Thiel P. G., Lübben A., Vleggaar R. Moniliformin production and toxicity of different Fusarium species from Southern Africa. Appl Environ Microbiol. 1982 Mar;43(3):517–521. doi: 10.1128/aem.43.3.517-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Ishiko T., Enomoto M., Otsubo K., Umeda M. Screening test using HeLa cells and mice for detection of mycotoxin-producing fungi isolated from foodstuffs. An additional report on fungi collected in 1968-1969. Jpn J Exp Med. 1974 Feb;44(1):63–82. [PubMed] [Google Scholar]
- Saito M., Otsubo K., Umeda M., Enomoto M., Kurata H. Screening tests using HeLa cells and mice for detection of mycotoxin-producing fungi isolated from foodstuffs. Jpn J Exp Med. 1971 Feb;41(1):1–20. [PubMed] [Google Scholar]
- Scott D. B. Toxigenic fungi isolated from cereal and legume products. Mycopathol Mycol Appl. 1965 Apr 14;25(3):213–222. doi: 10.1007/BF02049914. [DOI] [PubMed] [Google Scholar]
- Scott P. M. Assessment of quantitative methods for determination of trichothecenes in grains and grain products. J Assoc Off Anal Chem. 1982 Jul;65(4):876–883. [PubMed] [Google Scholar]
- URAGUCHI K. Isolation of two toxic agents, luteoskyrin and chlorine-containing peptide, from the metabolites of Penicillium islandicum Sopp, with some properties thereof. Jpn J Exp Med. 1961 Feb;31:19–46. [PubMed] [Google Scholar]
- Ueno Y., Ishikawa Y., Nakajima M., Sakai K., Ishii K. Toxicological approaches to the metabolites of Fusaria. I. Screening of toxic strains. Jpn J Exp Med. 1971 Aug;41(4):257–272. [PubMed] [Google Scholar]
- Yoshizawa T., Swanson S. P., Mirocha C. J. In vitro metabolism of T-2 toxin in rats. Appl Environ Microbiol. 1980 Nov;40(5):901–906. doi: 10.1128/aem.40.5.901-906.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T., Swanson S. P., Mirocha C. J. T-2 metabolites in the excreta of broiler chickens administered 3H-labeled T-2 toxin. Appl Environ Microbiol. 1980 Jun;39(6):1172–1177. doi: 10.1128/aem.39.6.1172-1177.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


