Abstract
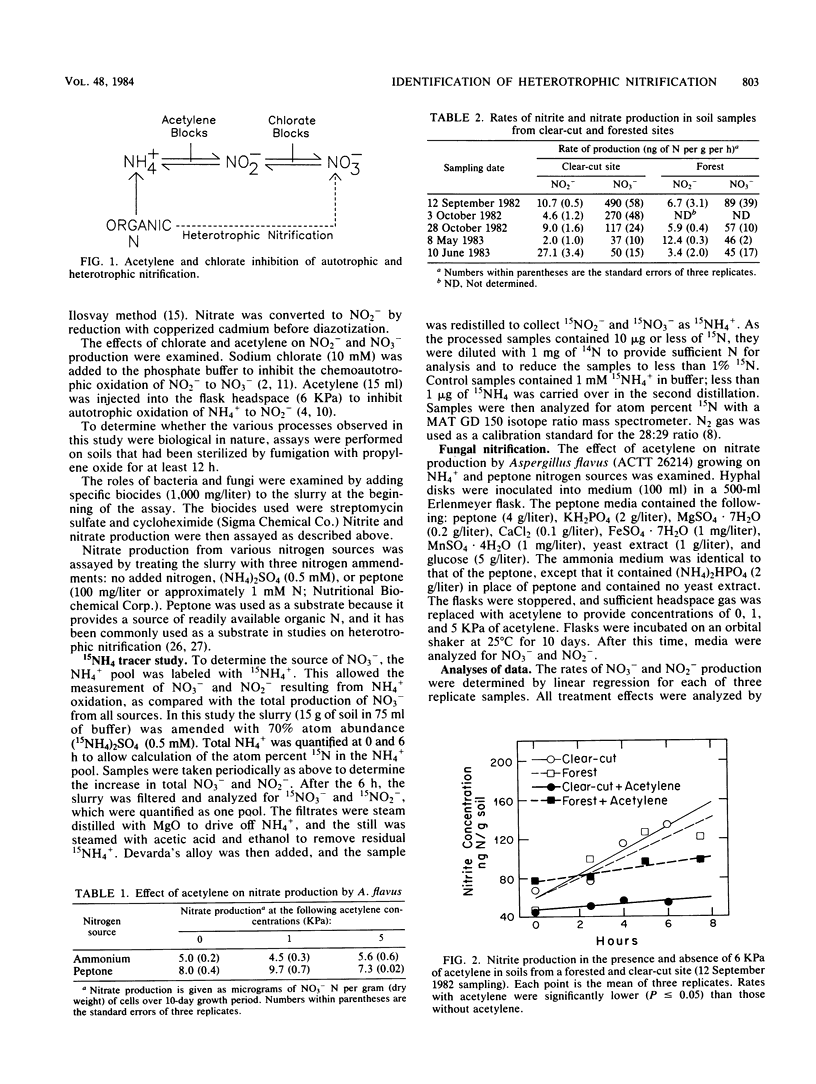
A potential for heterotrophic nitrification was identified in soil from a mature conifer forest and from a clear-cut site. Potential rates of NO2− production were determined separately from those of NO3− by using acetylene to block autotrophic NH4+ oxidation and chlorate to block NO2− oxidation to NO3− in soil slurries. Rates of NO2− production were similar in soil from the forest and the clear-cut site and were strongly inhibited by acetylene. The rate of NO3− production was much greater than that of NO2− production, and NO3− production was not significantly affected by acetylene or chlorate. Nitrate production was partially inhibited by cycloheximide, but was not significantly reduced by streptomycin. Neither the addition of ammonium nor the addition of peptone stimulated NO3− production. 15N labeling of the NH4+ pool demonstrated that NO3− was not coming from NH4+. The potential for heterotrophic nitrification in these forest soils was greater than that for autotrophic nitrification.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belser L. W., Mays E. L. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol. 1980 Mar;39(3):505–510. doi: 10.1128/aem.39.3.505-510.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Mays E. L. Use of nitrifier activity measurements to estimate the efficiency of viable nitrifier counts in soils and sediments. Appl Environ Microbiol. 1982 Apr;43(4):945–948. doi: 10.1128/aem.43.4.945-948.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W. Population ecology of nitrifying bacteria. Annu Rev Microbiol. 1979;33:309–333. doi: 10.1146/annurev.mi.33.100179.001521. [DOI] [PubMed] [Google Scholar]
- Hynes R. K., Knowles R. Inhibition of chemoautotrophic nitrification by sodium chlorate and sodium chlorite: a reexamination. Appl Environ Microbiol. 1983 Apr;45(4):1178–1182. doi: 10.1128/aem.45.4.1178-1182.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likens G. E., Bormann F. H., Johnson N. M. Nitrification: importance to nutrient losses from a cutover forested ecosystem. Science. 1969 Mar 14;163(3872):1205–1206. doi: 10.1126/science.163.3872.1205. [DOI] [PubMed] [Google Scholar]
- Tate R. L., 3rd Nitrification in histosols: a potential role for the heterotrophic nitrifier. Appl Environ Microbiol. 1977 Apr;33(4):911–914. doi: 10.1128/aem.33.4.911-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek P. M., Gosz J. R., Grier C. C., Melillo J. M., Reiners W. A., Todd R. L. Nitrate losses from disturbed ecosystems. Science. 1979 May 4;204(4392):469–474. doi: 10.1126/science.204.4392.469. [DOI] [PubMed] [Google Scholar]


