Abstract
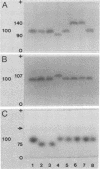
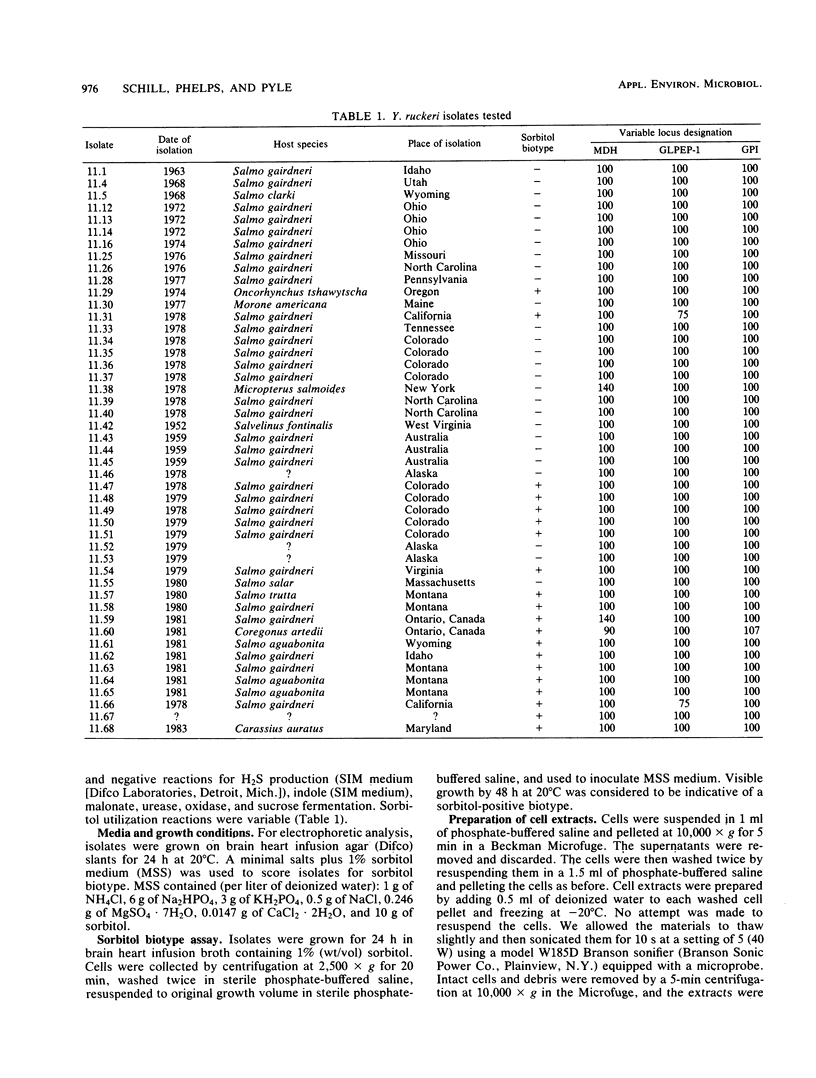
Multilocus isoenzyme electrophoresis was used to screen 47 field isolates of Yersinia ruckeri for electrophoretic variation at 15 enzyme loci. Only four electrophoretic types were observed, thus indicating that the genetic structure of Y. ruckeri is clonal. Forty-two isolates were of one electrophoretic type, a reflection of the low amount of genetic diversity extant in this species. Although sorbitol fermentation has been considered to be indicative of a second biotype, no significant gene frequency differences were found between the group of 20 isolates that readily used sorbitol as the sole carbon source and the group of 27 that did not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allendorf F. W., Mitchell N., Ryman N., Ståhl G. Isozyme loci in brown trout (Salmo trutta L.): detection and interpretation from population data. Hereditas. 1977;86(2):179–190. doi: 10.1111/j.1601-5223.1977.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman R. Electrophoretic variation in Escherichia coli from natural sources. Science. 1973 Dec 7;182(4116):1024–1026. doi: 10.1126/science.182.4116.1024. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Colwell R. R., Hetrick F. M. Characterization of plasmids in bacterial fish pathogen. Infect Immun. 1983 Jan;39(1):184–192. doi: 10.1128/iai.39.1.184-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam T. S., Ochman H., Selander R. K. Geographic components of linkage disequilibrium in natural populations of Escherichia coli. Mol Biol Evol. 1983 Dec;1(1):67–83. doi: 10.1093/oxfordjournals.molbev.a040302. [DOI] [PubMed] [Google Scholar]
- Whittam T. S., Ochman H., Selander R. K. Multilocus genetic structure in natural populations of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1751–1755. doi: 10.1073/pnas.80.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]