Abstract
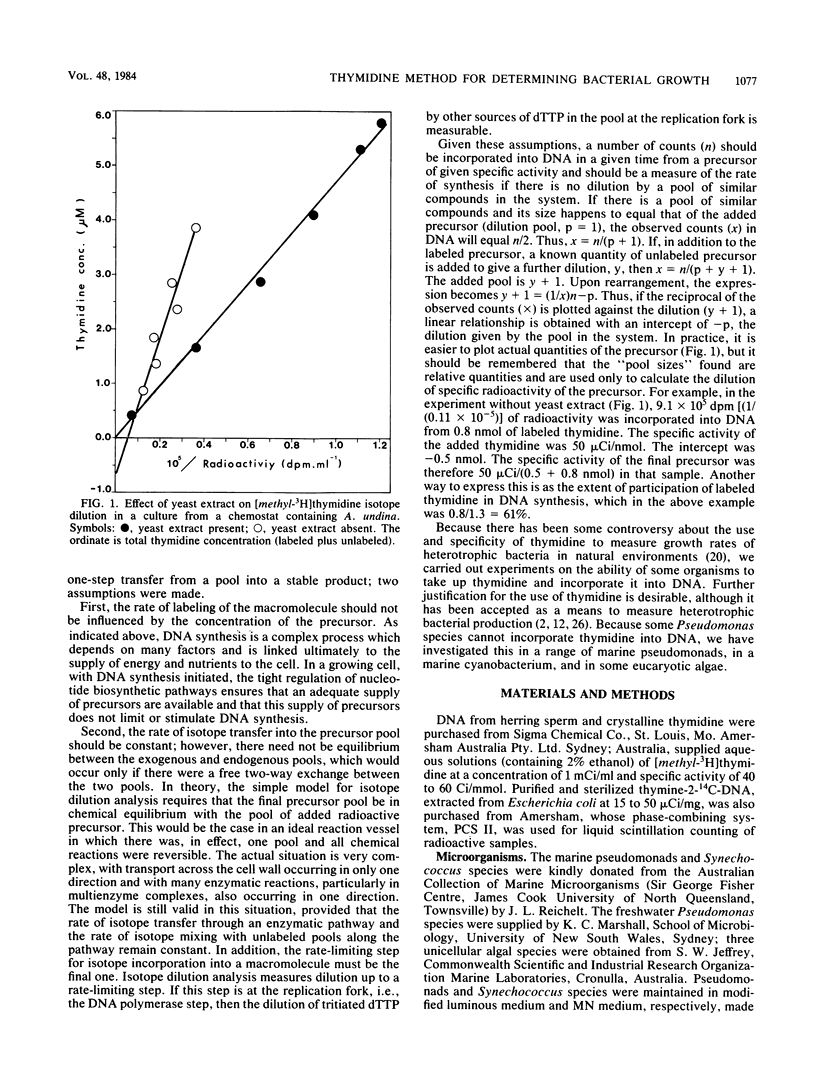
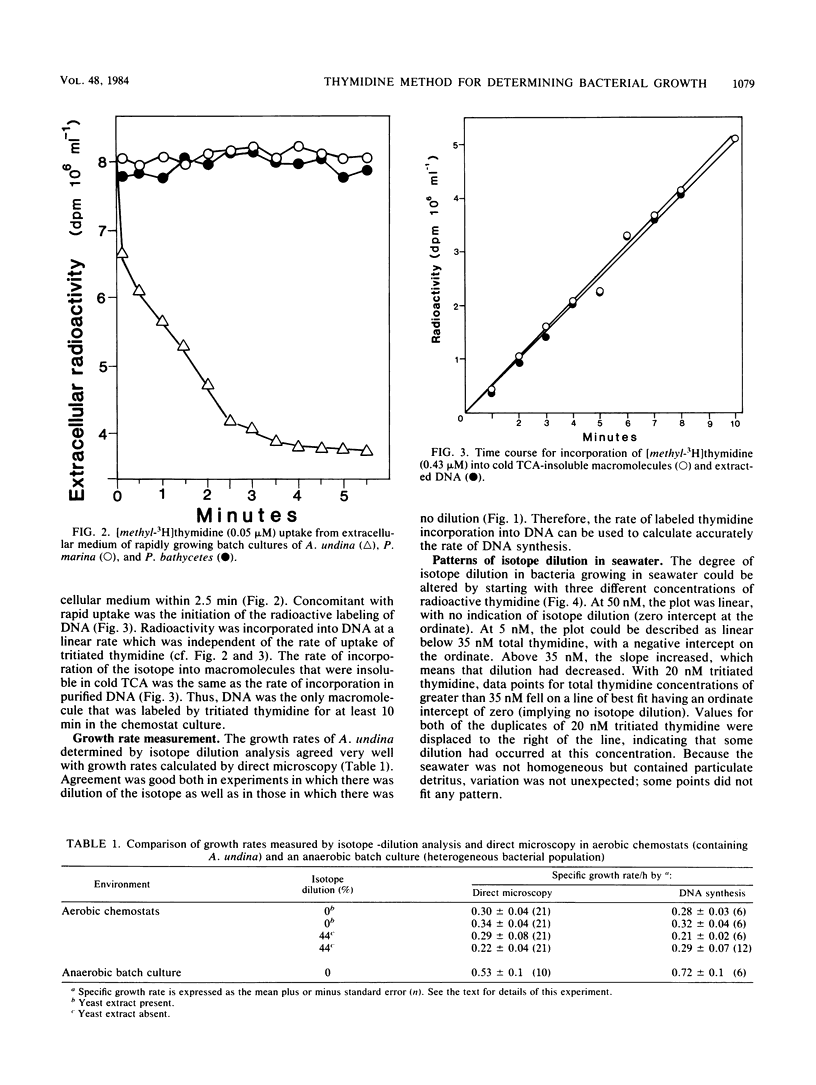
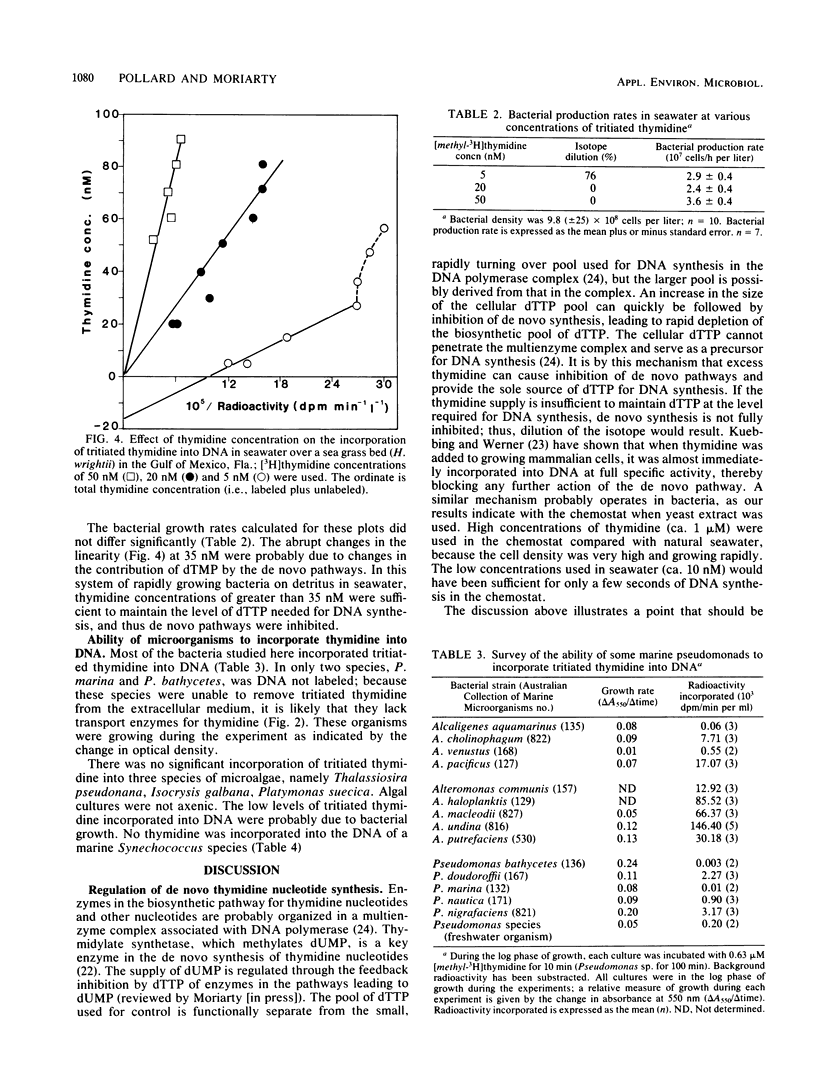
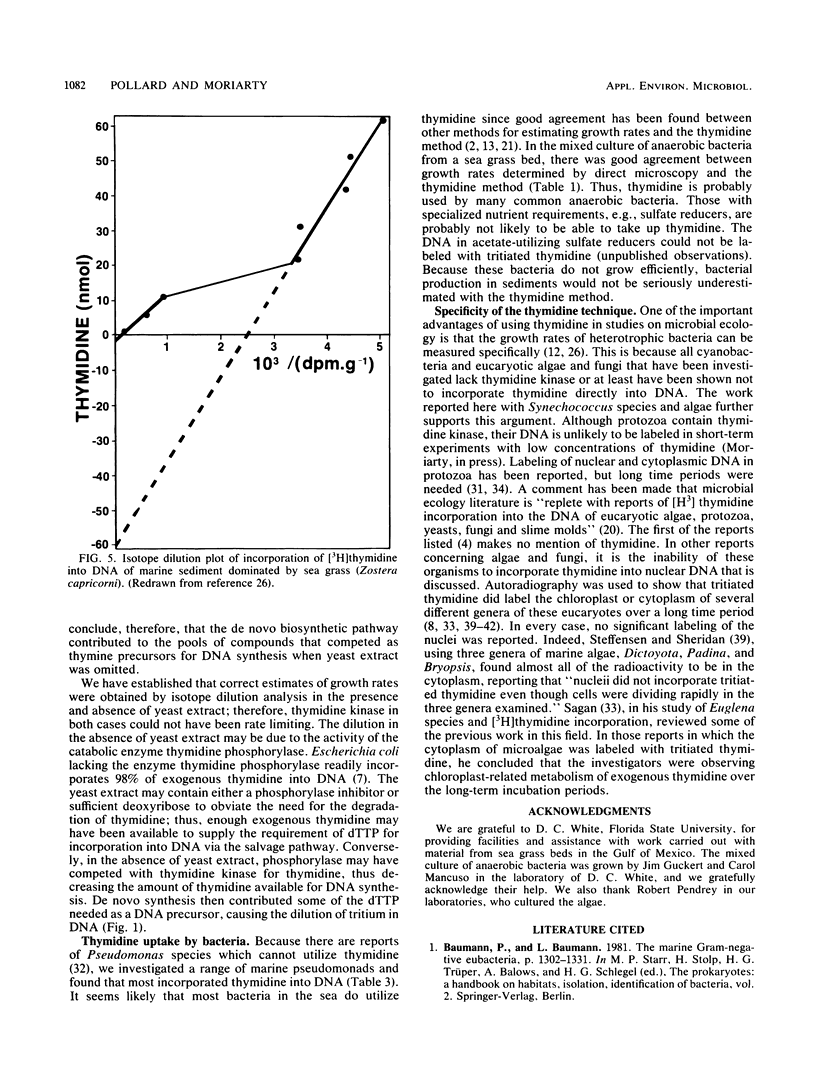
The rate of tritiated thymidine incorporation into DNA was used to estimate bacterial growth rates in aquatic environments. To be accurate, the calculation of growth rates has to include a factor for the dilution of isotope before incorporation. The validity of an isotope dilution analysis to determine this factor was verified in experiments reported here with cultures of a marine bacterium growing in a chemostat. Growth rates calculated from data on chemostat dilution rates and cell density agreed well with rates calculated by tritiated thymidine incorporation into DNA and isotope dilution analysis. With sufficiently high concentrations of exogenous thymidine, de novo synthesis of deoxythymidine monophosphate was inhibited, thereby preventing the endogenous dilution of isotope. The thymidine technique was also shown to be useful for measuring growth rates of mixed suspensions of bacteria growing anaerobically. Thymidine was incorporated into the DNA of a range of marine pseudomonads that were investigated. Three species did not take up thymidine. The common marine cyanobacterium Synechococcus species did not incorporate thymidine into DNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Microbial growth rates in nature. Bacteriol Rev. 1971 Mar;35(1):39–58. doi: 10.1128/br.35.1.39-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow H. W., Kirchman D. L., Rowe G. T. Production and vertical flux of attached bacteria in the hudson river plume of the new york bight as studied with floating sediment traps. Appl Environ Microbiol. 1982 Apr;43(4):769–776. doi: 10.1128/aem.43.4.769-776.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK R. M., FINK K. Relative retention of H3 and C14 labels of nucleosides incorporated into nucleic acids of Neurospora. J Biol Chem. 1962 Sep;237:2889–2891. [PubMed] [Google Scholar]
- Fangman W. L. Specificity and efficiency of thymidine incorporation in Escherichia coli lacking thymidine phosphorylase. J Bacteriol. 1969 Sep;99(3):681–687. doi: 10.1128/jb.99.3.681-687.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Application of the isotope-dilution principle to the analysis of factors affecting the incorporation of (3H) uridine and (3H) cytidine into cultured lymphocytes. Evaluation of pools in serum and culture media. Biochem J. 1971 Dec;125(3):721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Studies on the incorporation of [5-3H] uridine during activation and transformation of lymphocytes induced by phytohaemagglutinin. Dependence on the incorporation rate on uridine concentration at certain critical concentrations. Biochem J. 1968 Mar;107(2):197–205. doi: 10.1042/bj1070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Francke B. In vitro polyoma DNA synthesis: characterization of a system from infected 3T3 cells. J Virol. 1974 Jan;13(1):125–139. doi: 10.1128/jvi.13.1.125-139.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- Iwatsuki N., Okazaki R. Mechanism of regulation of deoxythymidine kinase of Escherichia coli. I. Effect of regulatory deoxynucleotides on the state of aggregation of the enzyme. J Mol Biol. 1967 Oct 14;29(1):139–154. doi: 10.1016/0022-2836(67)90186-6. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Okazaki R. Mechanism of regulation of deoxythymidine kinase of Escherichia coli. II. Effect of temperature on the enzyme activity and kinetics. J Mol Biol. 1967 Oct 14;29(1):155–165. doi: 10.1016/0022-2836(67)90187-8. [DOI] [PubMed] [Google Scholar]
- Karl D. M. Selected nucleic Acid precursors in studies of aquatic microbial ecology. Appl Environ Microbiol. 1982 Oct;44(4):891–902. doi: 10.1128/aem.44.4.891-902.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebbing D., Werner R. A model for compartmentation of de novo and salvage thymidine nucleotide pools in mammalian cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3333–3336. doi: 10.1073/pnas.72.9.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- PLAUT W., SAGAN L. A. Incorporation of thymidine in the cytoplasm of Amoeba proteus. J Biophys Biochem Cytol. 1958 Nov 25;4(6):843–846. doi: 10.1083/jcb.4.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGAN L. AN UNUSUAL PATTERN OF TRITIATED THYMIDINE INCORPORATION IN EUGLENA. J Protozool. 1965 Feb;12:105–109. doi: 10.1111/j.1550-7408.1965.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Scott F. W., Forsdyke D. R. Isotope-dilution analysis of the effects of deoxyguanosine and deoxyadenosine on the incorporation of thymidine and deoxycytidine by hydroxyurea-treated thymus cells. Biochem J. 1980 Sep 15;190(3):721–730. doi: 10.1042/bj1900721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott F. W., Forsdyke D. R. Isotope-dilution studies of the effects of 5-fluorodeoxyuridine and hydroxyurea on the incorporation of deoxycytidine and thymidine by cultured thymus cells. Can J Biochem. 1976 Mar;54(3):238–248. doi: 10.1139/o76-037. [DOI] [PubMed] [Google Scholar]
- Scott F. W., Forsdyke D. R. The rate of deoxyribonucleic acid synthesis by cultured Chinese-hamster ovary cells. An application of isotope-dilution analysis. Biochem J. 1978 Mar 15;170(3):545–549. doi: 10.1042/bj1700545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom D. A., Forsdyke D. R. Isotope-dilution analysis of rate-limiting steps and pools affecting the incorporation of thymidine and deoxycytidine into cultured thymus cells. Biochem J. 1974 Feb;138(2):253–262. doi: 10.1042/bj1380253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen D. M., Sheridan W. F. Incorporation of H3-thymidine into chloroplast DNA of marine algae. J Cell Biol. 1965 Jun;25(3):619–626. doi: 10.1083/jcb.25.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinton D. C., Hanawalt P. C. In vivo specific labeling of Chlamydomonas chloroplast DNA. J Cell Biol. 1972 Sep;54(3):592–597. doi: 10.1083/jcb.54.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]


