Abstract
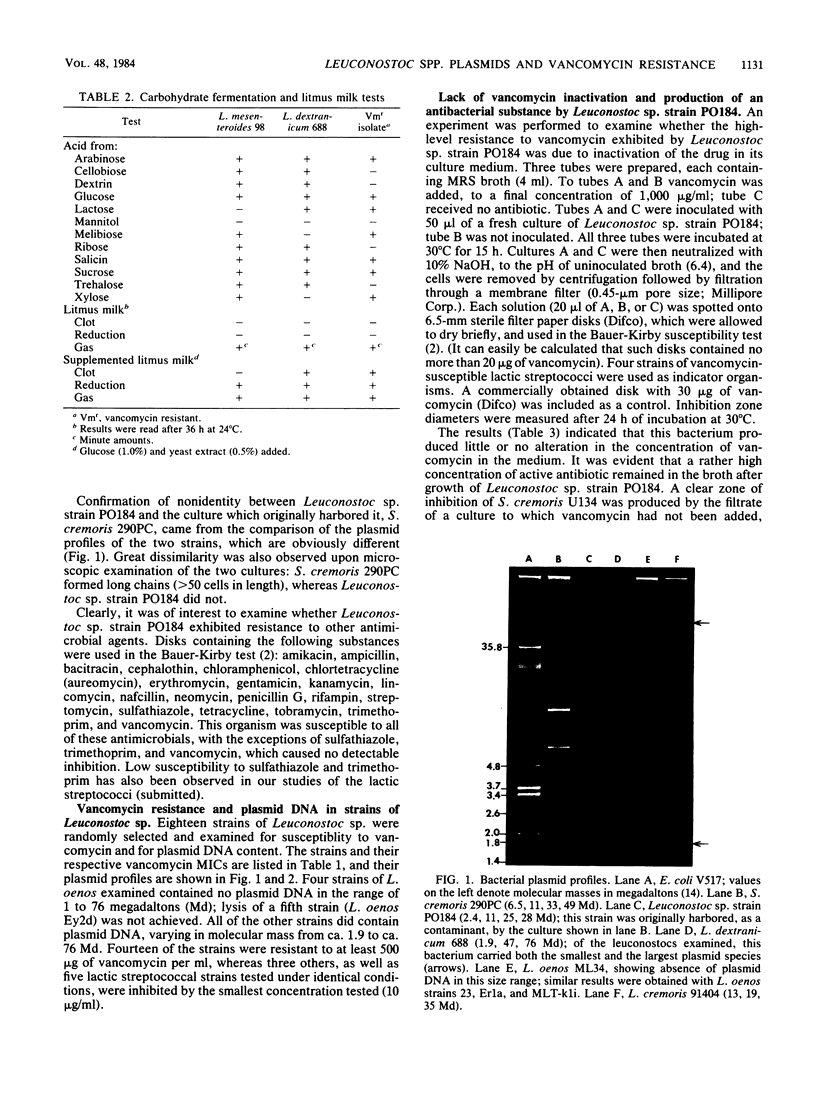
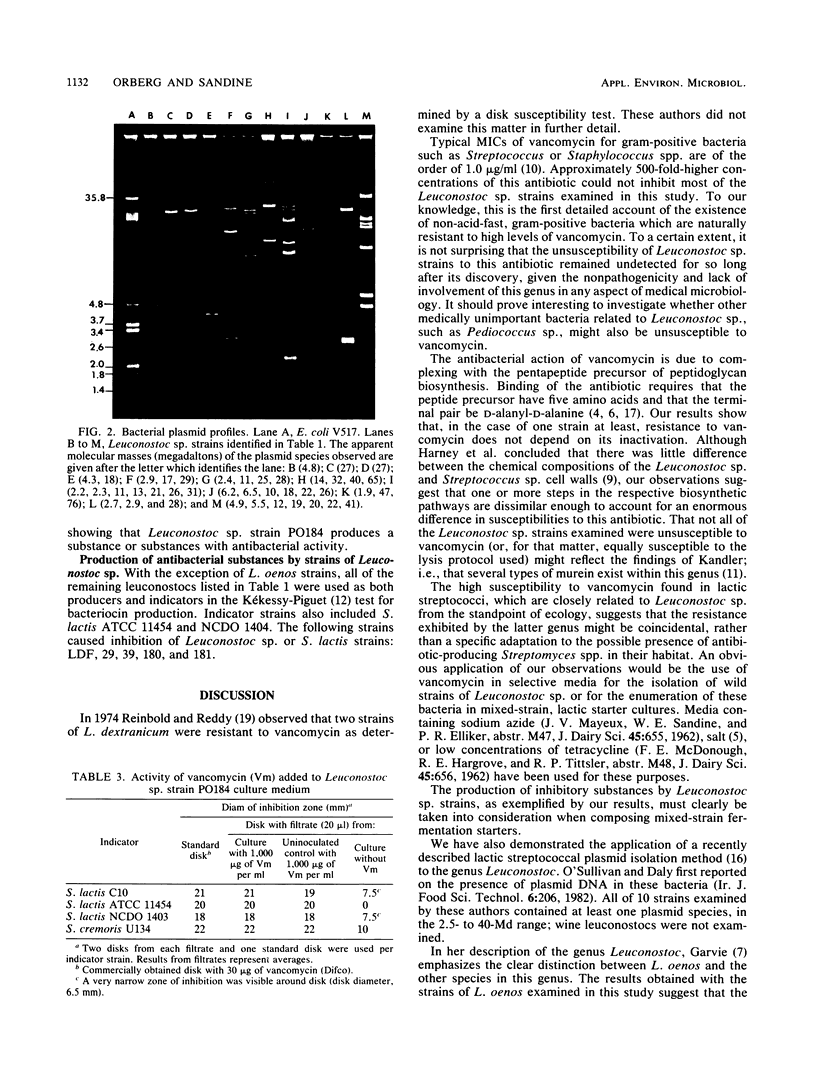
Resistance to vancomycin permitted detection, in a culture of Streptococcus cremoris 290PC, of a contaminant gram-positive coccus. Morphological and physiological characteristics indicated that this bacterium was a strain of Leuconostoc sp., designated PO184. This strain contained four plasmid species, which were distinct from those harbored by S. cremoris 290PC. Antibiotic disk susceptibility tests indicated that Leuconostoc sp. strain PO184 was also resistant to sulfathiazole and trimethoprim and susceptible to 17 other antimicrobials. The MIC of vancomycin for this strain was greater than 2,000 micrograms/ml, and resistance did not depend on drug inactivation. Leuconostoc sp. strain PO184 produced a substance which was inhibitory to S. cremoris U134, but not to S. lactis ATCC 11454. Five other leuconostocs produced substances with antibacterial activity. Of 18 strains of Leuconostoc sp., 14 were resistant to at least 500 micrograms of vancomycin per ml, including four L. oenos strains which harbored no plasmid DNA in the 1- to 76-megadalton range. Twelve Leuconostoc sp. strains contained at least one plasmid species in this mass range. These findings are discussed from the physiological, taxonomical, and ecological standpoints and with regard to their potential applications.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harney S. J., Simopoulos N. D., Ikawa M. Cell wall constituents of Leuconostoc citrovorum and Leuconostoc mesenteroides. J Bacteriol. 1967 Jan;93(1):273–277. doi: 10.1128/jb.93.1.273-277.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kékessy D. A., Piguet J. D. New method for detecting bacteriocin production. Appl Microbiol. 1970 Aug;20(2):282–283. doi: 10.1128/am.20.2.282-283.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Orberg P. K., Sandine W. E. Microscale method for rapid isolation of covalently closed circular plasmid DNA from group N streptococci. Appl Environ Microbiol. 1984 Apr;47(4):677–680. doi: 10.1128/aem.47.4.677-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. Vancomycin and related antibiotics. Pharmacol Ther. 1982;16(2):181–197. doi: 10.1016/0163-7258(82)90053-5. [DOI] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




