Abstract
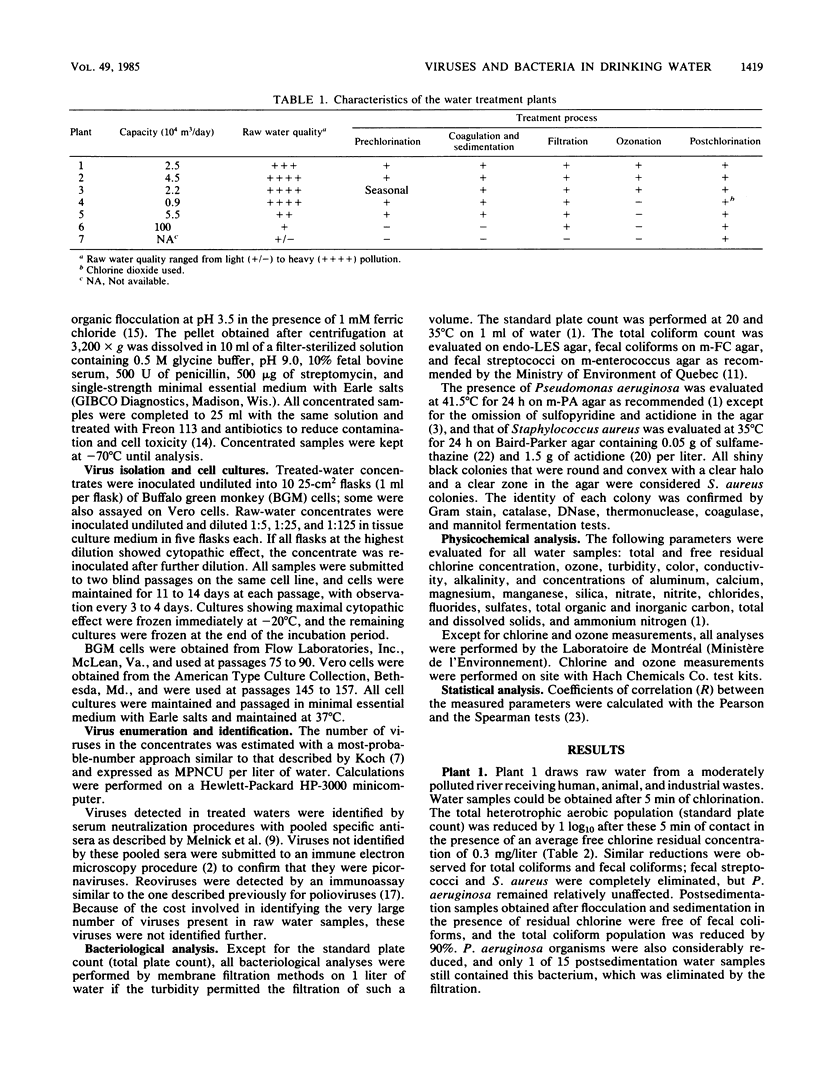
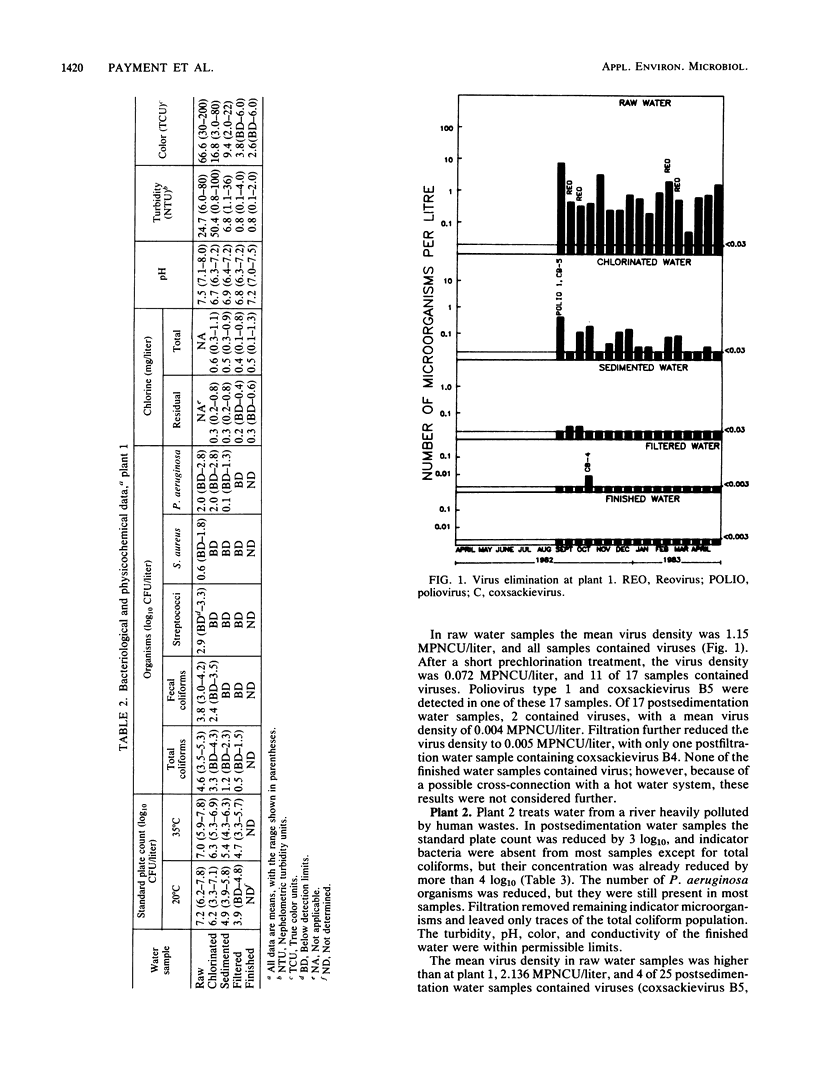
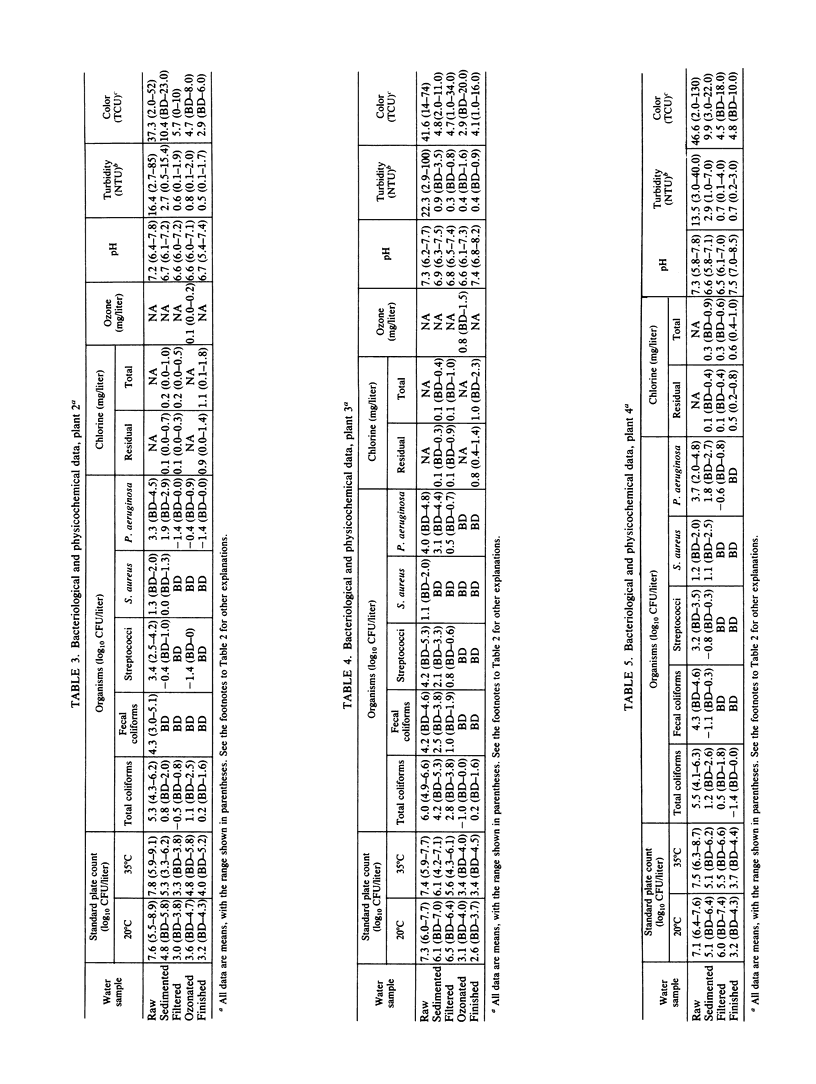
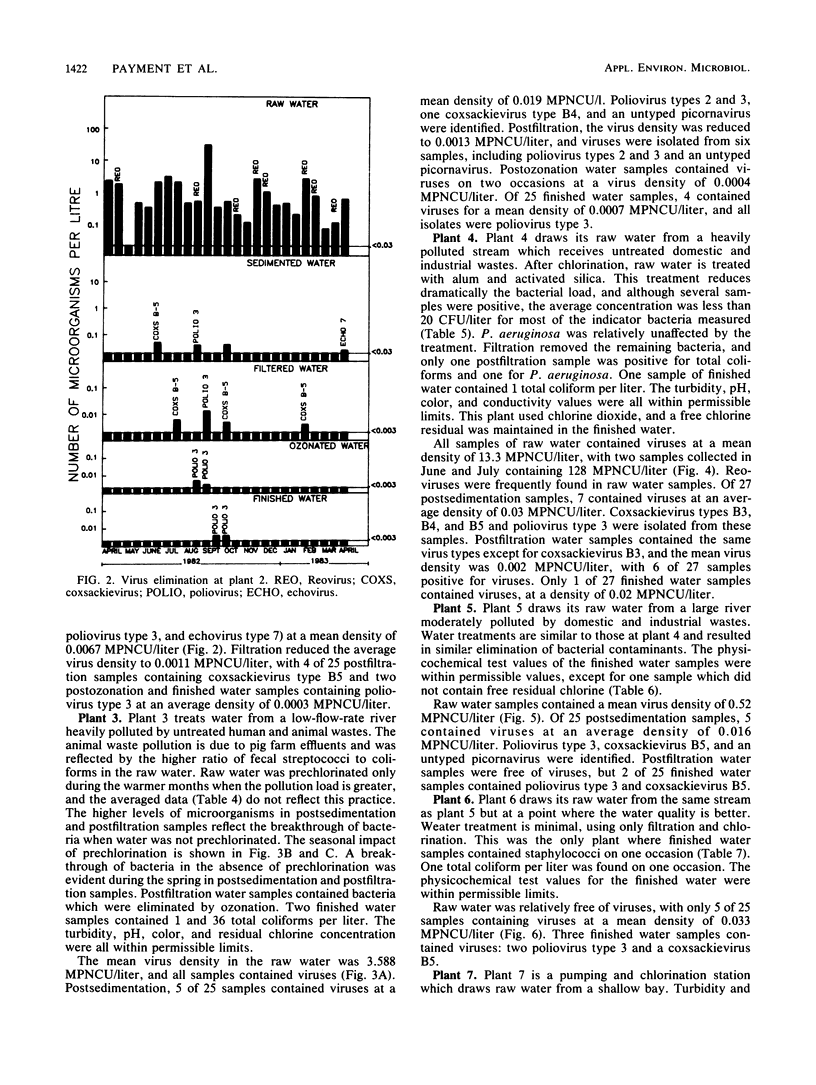
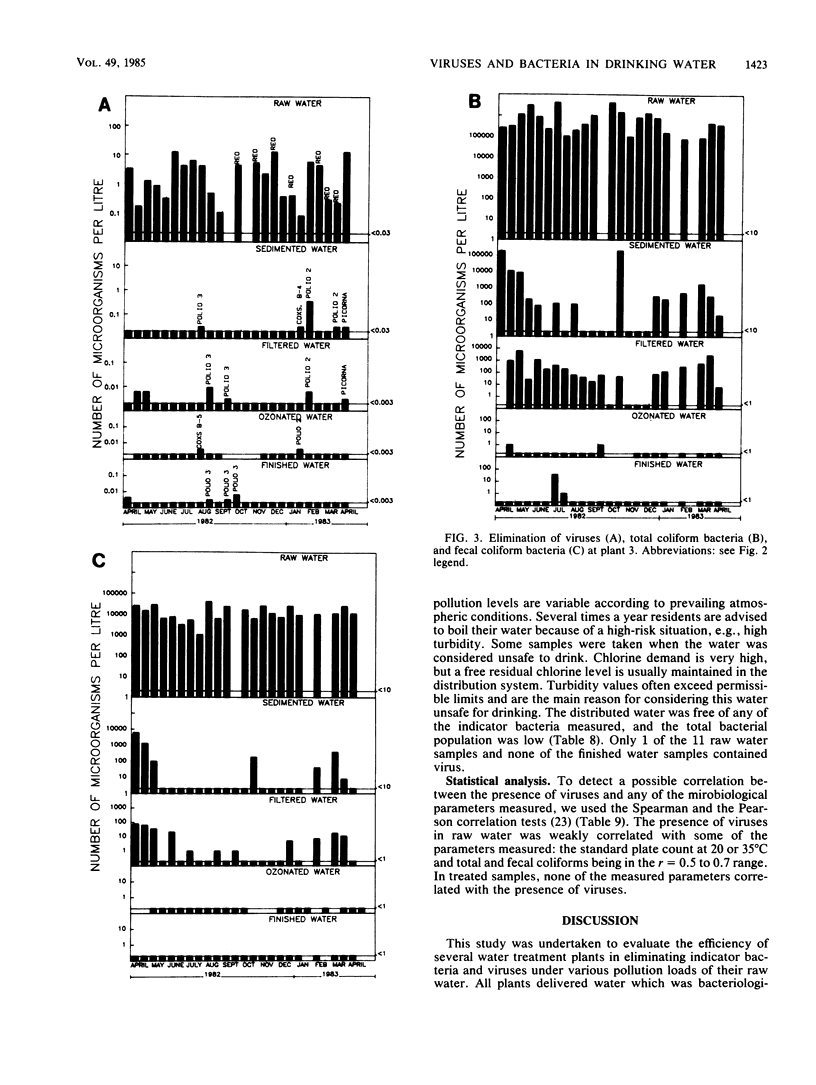
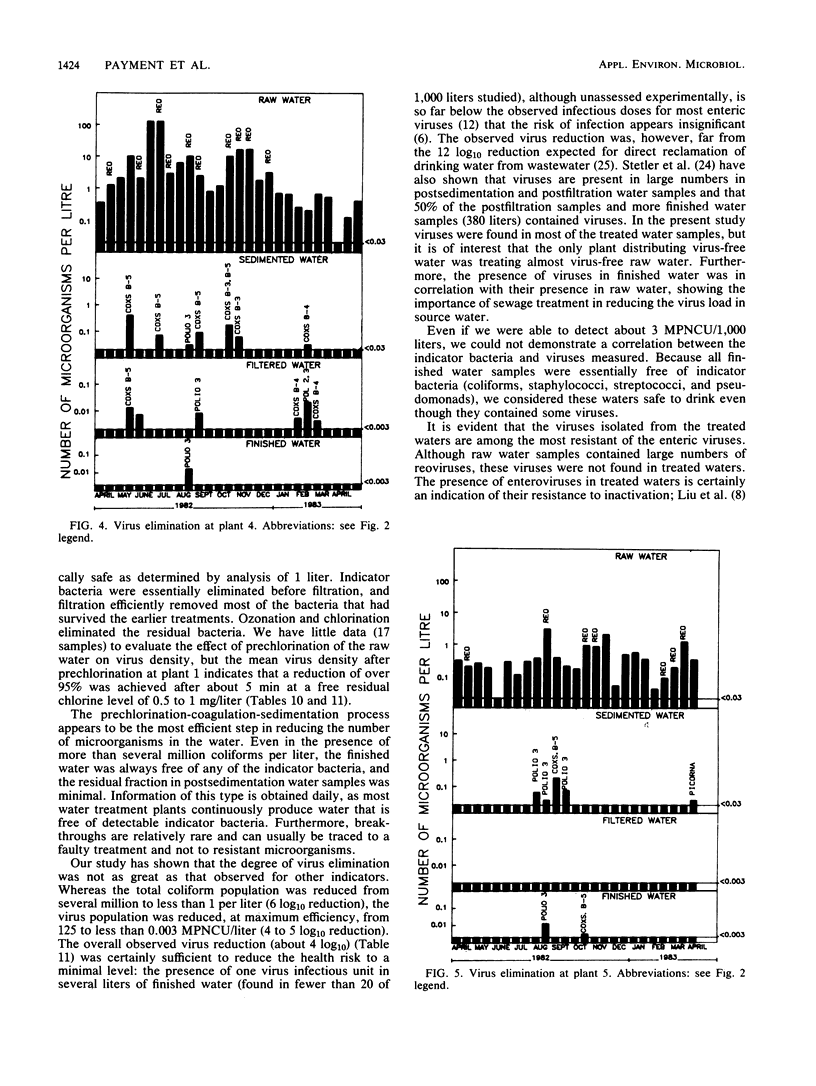
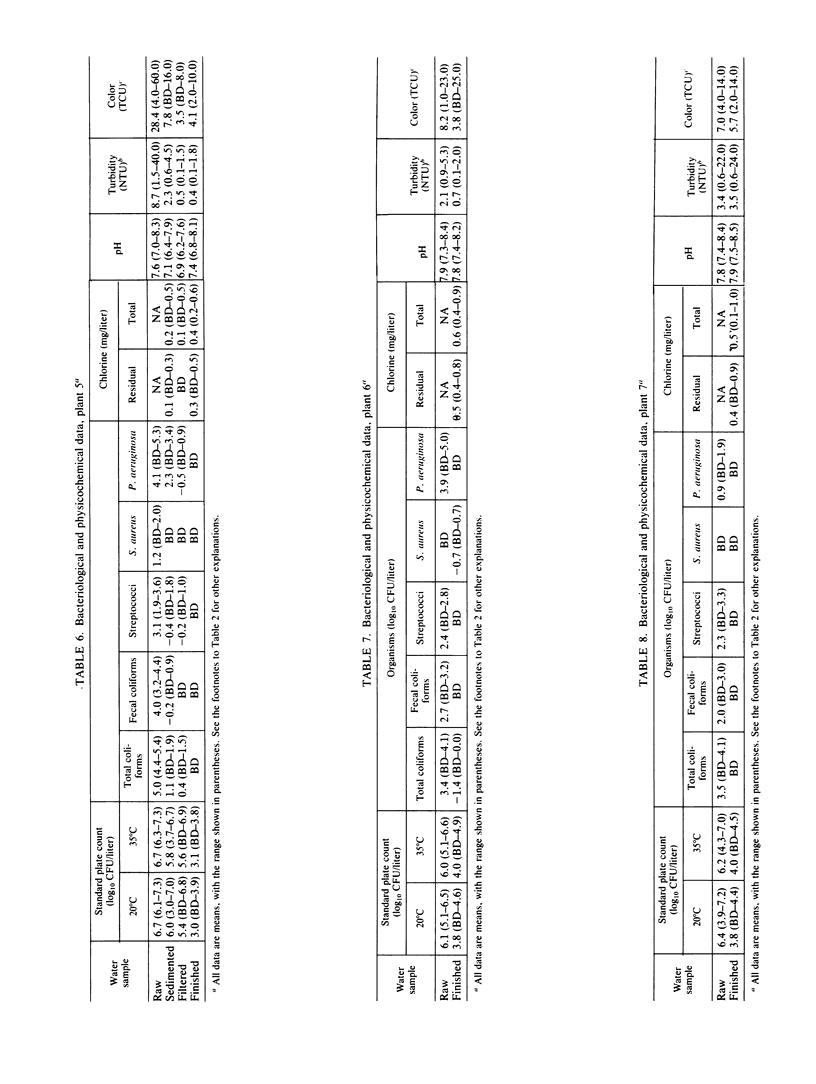
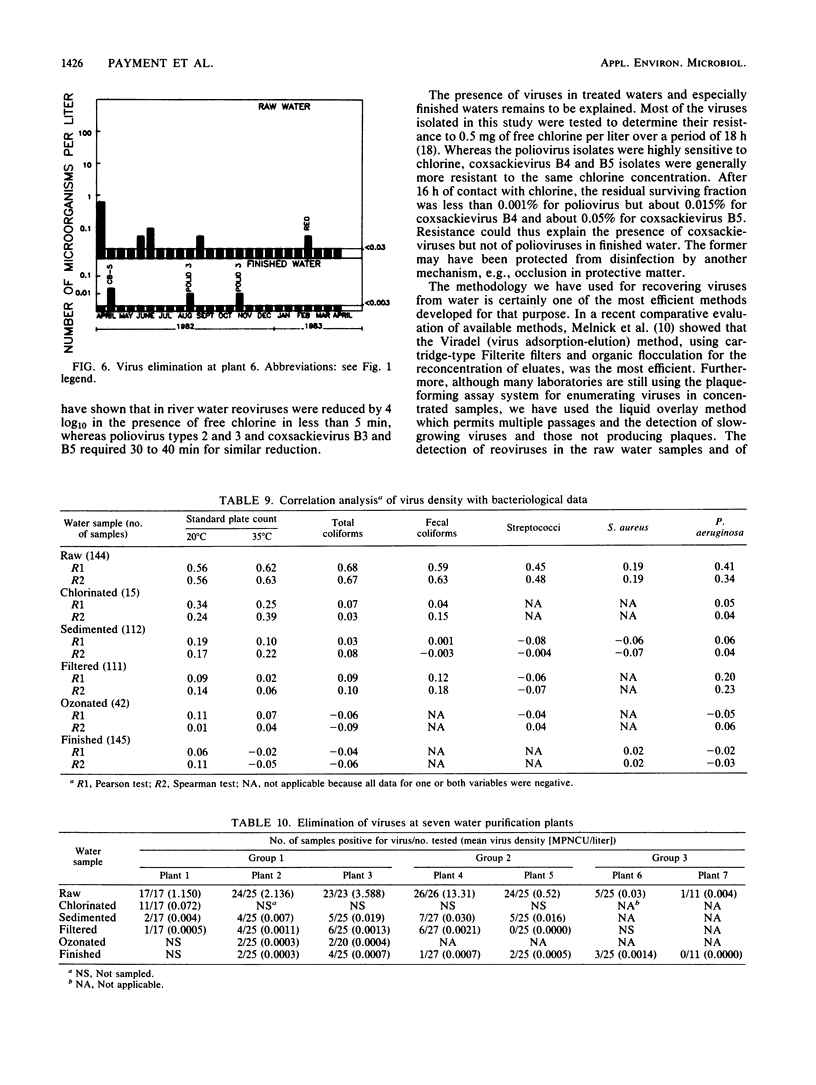
Seven drinking water treatment plants were sampled twice a month for 12 months to evaluate the removal of indicator bacteria and cytopathogenic enteric viruses. Samples were obtained at each level of treatment: raw water, postchlorination, postsedimentation, postfiltration, postozonation, and finished (tap) water. Raw water quality was usually poor, with total coliform counts exceeding 105 to 106 CFU/liter and the average virus count in raw water of 3.3 most probable number of cytopathogenic units (MPNCU)/liter; several samples contained more than 100 MPNCU/liter. All plants distributed finished water that was essentially free of indicator bacteria as judged by analysis of 1 liter for total coliforms, fecal coliforms, fecal streptococci, coagulase-positive staphylococci, and Pseudomonas aeruginosa. The total plate counts at 20 and 35 degrees C were also evaluated as a measure of the total microbial population and were usually very low. Viruses were detected in 7% (11 of 155) of the finished water samples (1,000 liters) at an average density of 0.0006 MPNCU/liter the highest virus density measured being 0.2 MPNCU/liter. The average cumulative virus reduction was 95.15% after sedimentation and 99.97% after filtration and did not significantly decrease after ozonation or final chlorination. The viruses isolated from treated waters were all enteroviruses: poliovirus types 1, 2, and 3, coxsackievirus types B3, B4, and B5, echovirus type 7, and untyped picornaviruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthiaume L., Alain R., McLaughlin B., Payment P., Trépanier P. Rapid detection of human viruses in faeces by a simple and routine immune electron microscopy technique. J Gen Virol. 1981 Jul;55(Pt 1):223–227. doi: 10.1099/0022-1317-55-1-223. [DOI] [PubMed] [Google Scholar]
- Brodsky M. H., Ciebin B. W. Improved medium for recovery and enumeration of Pseudomonas aeruginosa from water using membrane filters. Appl Environ Microbiol. 1978 Jul;36(1):36–42. doi: 10.1128/aem.36.1.36-42.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Estimation of the most probable number with a programable pocket calculator. Appl Environ Microbiol. 1982 Feb;43(2):488–490. doi: 10.1128/aem.43.2.488-490.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Rennick V., Hampil B., Schmidt N. J., Ho H. H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull World Health Organ. 1973;48(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Safferman R., Rao V. C., Goyal S., Berg G., Dahling D. R., Wright B. A., Akin E., Stetler R., Sorber C. Round robin investigation of methods for the recovery of poliovirus from drinking water. Appl Environ Microbiol. 1984 Jan;47(1):144–150. doi: 10.1128/aem.47.1.144-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor T. E., Allen C. I., Tsiatis A. A., Nelson D. B., D'Alessio D. J. Human infective dose determinations for oral poliovirus type 1 vaccine in infants. J Clin Microbiol. 1981 Feb;13(2):388–389. doi: 10.1128/jcm.13.2.388-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P., Ayache R., Trudel M. A survey of enteric viruses in domestic sewage. Can J Microbiol. 1983 Jan;29(1):111–119. doi: 10.1139/m83-018. [DOI] [PubMed] [Google Scholar]
- Payment P., Fortin S., Trudel M. Ferric chloride flocculation for nonflocculating beef extract preparations. Appl Environ Microbiol. 1984 Mar;47(3):591–592. doi: 10.1128/aem.47.3.591-592.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P. Isolation of viruses from drinking water at the Point-Viau water treatment plant. Can J Microbiol. 1981 Apr;27(4):417–420. doi: 10.1139/m81-063. [DOI] [PubMed] [Google Scholar]
- Payment P., Tremblay C., Trudel M. Rapid identification and serotyping of poliovirus isolates by an immunoassay. J Virol Methods. 1982 Dec;5(5-6):301–308. doi: 10.1016/0166-0934(82)90021-0. [DOI] [PubMed] [Google Scholar]
- Payment P., Tremblay M., Trudel M. Relative resistance to chlorine of poliovirus and coxsackievirus isolates from environmental sources and drinking water. Appl Environ Microbiol. 1985 Apr;49(4):981–983. doi: 10.1128/aem.49.4.981-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P., Trudel M., Sattar S. A., Springthorpe V. S., Subrahmanyan T. P., Gregory B. E., Vajdic A. H., Blaskovic P., Guglielmi I. J., Kudrewko O. Virological examination of drinking water: a Canadian collaborative study. Can J Microbiol. 1984 Jan;30(1):105–112. doi: 10.1139/m84-018. [DOI] [PubMed] [Google Scholar]
- Sekla L., Stackiw W., Kay C., VanBuckenhout L. Enteric viruses in renovated water in Manitoba. Can J Microbiol. 1980 Apr;26(4):518–523. doi: 10.1139/m80-087. [DOI] [PubMed] [Google Scholar]
- Stetler R. E., Ward R. L., Waltrip S. C. Enteric virus and indicator bacteria levels in a water treatment system modified to reduce trihalomethane production. Appl Environ Microbiol. 1984 Feb;47(2):319–324. doi: 10.1128/aem.47.2.319-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


