Abstract
A deficiency in essential fatty acid metabolism has been reported in plasma from patients with cystic fibrosis (CF). However, its etiology and role in the expression of disease is unknown. The objective of this study was to determine whether alterations in fatty acid metabolism are specific to CF-regulated organs and whether they play a role in the expression of disease. A membrane lipid imbalance was found in ileum, pancreas, and lung from cftr−/− mice characterized by an increase in phospholipid-bound arachidonic acid and a decrease in phospholipid-bound docosahexaenoic acid (DHA). This lipid imbalance was observed in organs pathologically affected by CF including lung, pancreas, and ileum and was not secondary to impaired intestinal absorption or hepatic biosynthesis of DHA. As proof of concept, oral administration of DHA to cftr−/− mice corrected this lipid imbalance and reversed the observed pathological manifestations. These results strongly suggest that certain phenotypic manifestations of CF may result from remediable alterations in phospholipid-bound arachidonic acid and DHA levels.
Keywords: docosahexaenoic acid, arachidonic acid, fish oil, pancreas, lung
Cystic fibrosis (CF) is the most prevalent lethal autosomal recessive disorder in the Caucasian population, affecting 1 in 2,500 newborns (1). Patients with CF express a typical phenotype characterized by pancreatic insufficiency, ileal hypertrophy, and recurrent pulmonary infections that ultimately lead to pulmonary failure and death. In 1989, the gene whose mutation results in CF was identified and cloned (2, 3). The product of the gene, the CF transmembrane conductance regulator (CFTR), was characterized as an ATP-gated chloride channel that is regulated by cAMP-dependent protein kinase phosphorylation (4).
Despite the significant advances made in CF research in recent years, the mechanism by which a mutation in the CFTR gene leads to the manifestations of this disease remains unclear. Although a decrease in apical membrane CFTR-dependent chloride conductance might explain some of the pathological manifestations observed in CF, e.g., viscous secretions, it explains neither the increased inflammation in the lung nor the membrane-recycling defects observed in CF (5–7).
Arachidonic acid (AA), an agonist of inflammatory pathways and a stimulant of mucus secretion, is elevated in the phospholipid fraction from bronchial alveolar lavage fluid in CF patients (5). However, the increased inflammation and elevated AA levels observed in CF have long been thought to be secondary to infection (8). This conclusion has been challenged recently by Heeckeren et al. (9), who demonstrated that instillation of agarose beads coated with Pseudomonas into the lungs of cftr−/− mice resulted in increased inflammation and mortality compared with that observed in wild-type mice. These findings suggest that the lungs of cftr−/− mice are primed for inflammation and that the increase in AA and inflammation observed in cftr−/− mice may be a primary event and not secondary to infection.
Docosahexaenoic acid (DHA), the end product of the n-3 pathway, is known to down-regulate AA incorporation into membrane phospholipids and to play a major role in regulating membrane fluidity and membrane trafficking (10–12). AA, a major intermediate of the n-6 pathway, is known to compete with DHA for the same elongation and desaturation enzymes and for the site of esterification at the sn-2 position of phospholipids. Although AA levels in membrane phospholipids can be decreased significantly by the administration of DHA or eicosapentaenoic acid (EPA), there is no known condition in which membrane-bound AA levels are increased above basal levels (13).
In this study, we present evidence that cftr−/− mice exhibit a marked imbalance in phospholipid-bound AA and DHA in organs clinically affected by CF including pancreas, intestine, and lung and that oral administration of DHA corrects this membrane lipid imbalance and normalizes the histology in ileum and pancreas.
Materials and Methods
Breeding of cftr−/− Mice and Oral Administration of DHA.
Experiments were carried out under protocols approved by the Beth Israel Deaconess Medical Center Animal Care Committee. A breeding colony was established by using University of North Carolina (UNC) heterozygous CFTR(+/−), exon 10 knockout mice (The Jackson Laboratory). Tail-clip samples of 14-day-old mice were processed for analysis of genotype as described (14). Both wild-type (C57) and cftr−/− mice were weaned at an average of 23 days of age. After weaning, mice were placed on water and Peptamen (Nestle Clinical Nutrition, Deerfield, IL) ad lib until 30 days of age and then continued for 7 days either with Peptamen or with 0.5, 2, 10, or 40 mg per day of either free or esterified DHA (Sigma) prepared as a stable emulsion in Peptamen. The volume of Peptamen administered was measured on a daily basis by using specific feeders. In a separate set of experiments, cftr−/− and wild-type mice also were fed 40 mg/day of α-linolenic acid or EPA for 7 days.
Cell and Tissue Preparation.
Mice were euthanized with carbon dioxide and blood was removed by cardiac puncture. Cell suspensions were prepared from pancreas and lung. Pancreatic acini were isolated by collagenase and mechanical dissociation (15). A total lung cell suspension was prepared by flushing contaminating blood with Krebs–Henseleit buffer (KHB), pH 7.4, containing 0.5% BSA. Tissue then was minced and incubated for 30 min at 37°C in 10 ml of oxygenated KHB containing 1,000 units of collagenase (Worthington), 2,000 units of DNase, and 0.5 units of thermolysin (Sigma). The cell suspension was sedimented once through KHB containing 4% BSA and washed once in KHB. Brain, kidney, and heart were removed and sonicated in 0.5 ml of 0.3 M sucrose at 4°C. For ileum, mucosal scrapings were used for sonication.
Analysis of AA and DHA by GC/MS.
Lipids were extracted from cell suspensions, tissue homogenates, and blood plasma with 6 vol of chloroform/methanol (2:1, vol/vol) and methylated (16). In some experiments, lipids also were fractionated into cholesterol esters, triglycerides, free fatty acids, and phospholipids by aminopropyl column chromatography, and fatty acid methyl esters were prepared (17). Fatty acid methyl esters were analyzed by using a Hewlett–Packard GC/MS mounted with a WCOT capillary column (Supelco-wax-10, 30 m × 0.53 mm i.d., 1-μm film thickness) (16). Fatty acids bound to the sn-2 position of phospholipids were analyzed by GC/MC after hydrolysis of the phospholipid fraction obtained by aminopropyl column with phospholipase A2 (16).
Analysis of Lung Inflammation.
Weight-matched wild-type and cftr−/− mice with and without pretreatment with oral DHA (40 mg/day) were given a single dose of aerosolized Pseudomonas lipopolysaccharide (LPS) (10 mg/15 g body weight) over 15 min once each day for 3 days. Animals were sacrificed 3 hr after receiving the last dose of Pseudomonas LPS on day 3, bronchoalveolar lavage (BAL) was performed, and neutrophil concentration was determined by microscopic analysis using a calibrated grid. Neutrophils were positively identified by tetramethylbenzidine staining.
Morphologic Studies.
Sample preparation.
For sample preparation, 1–2 cm of ileum adjacent to the cecum was fixed in formalin, embedded in paraffin, cut in cross-section, and stained with hematoxylin/eosin. For pancreatic analyses, 2- to 3-mm pieces of pancreas were fixed for 1.5 hr in 2% glutaraldehyde in 0.2 M cacodylate buffer, pH 7.4, at room temperature. Tissue samples then were washed in 0.2 M cacodylate buffer and postfixed for 1 hr in 1% osmium tetroxide in 0.1 M cacodylate buffer. Tissue was embedded in epon and thick sections were stained with 1% toluidine blue.
Morphometry.
For measurement of ileal villus height or pancreatic luminal diameter, multiple images were taken randomly at ×200 by using a Nikon Microphot-SA microscope equipped with a Diagnostic Instruments Spot Digitizing System. Images were examined by two independent observers blinded to the conditions. Villi height in the ileum was quantitated by using nih image software. For the pancreas, all images were printed at the same magnification and luminal diameter was measured. A minimum of 12 images were analyzed from each animal.
Statistical Analysis.
The differences between the means in the different groups tested were evaluated by using the Student’s t test.
Results
Phospholipid-Bound AA Is Increased and Phospholipid-Bound DHA Is Decreased in Pancreas, Lung, and Ileum from cftr−/− Mice.
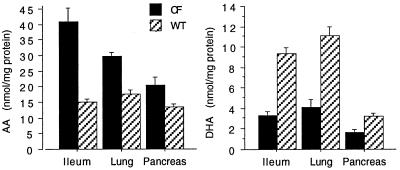
Because pancreas, lung, and ileum are the principal organs clinically affected in CF, AA and DHA levels in cell preparations of these organs in wild-type and cftr−/− mice were quantitated by GC/MS analysis (Fig. 1). One difficulty in studying these cftr−/− mice is the variable expression of disease (18). To overcome this, only mice weighing 7–9 g at day 23 of age, representing a more consistent phenotype, were used for experiments. Mucosal scrapings of ileum from cftr−/− mice demonstrated a 3-fold increase in AA (P = 0.005) and a 3-fold decrease in DHA (P = 0.001) compared with wild-type mice. Similarly, in total lung cell as well as pancreatic acini preparations, we observed a significant increase in AA (P < 0.01) and a marked decrease in DHA (P < 0.0001) in cftr−/− mice compared with wild-type controls. As expected, greater than 85% of the AA and DHA in these organs was esterified to the sn-2 position of phospholipids as determined by aminopropyl column chromatography, enzymatic hydrolysis with phospholipase A2, and GC/MS. With the exception of a 2-fold increase in phospholipid-bound docosatetranoic acid (22:4n-6) observed in pancreas and lung from cftr−/− mice (data not shown), no other significant lipid changes in cftr−/− mice compared with wild-type controls were detected. Because the cell preparations from pancreas and lung are devoid of the thick, extracellular secretions characteristic of CF, phospholipid-bound AA and DHA values correspond to levels in membranes from these epithelial cells and not from secretions from the extracellular space.
Figure 1.
AA and DHA levels in ileum, lung, and pancreas from cftr−/− and wild-type mice. AA and DHA levels were quantitated from the respective organs from cftr−/− (CF) and wild-type (WT) mice and expressed as nmol/mg protein. Results are expressed as the means ± SD (n = 7).
One possible explanation for these changes in AA and DHA is that protein content is different between wild-type and cftr−/− mice. Therefore, the data also were calculated in terms of mol percent of AA and DHA relative to total membrane fatty acid content. In pancreas, AA was 11.5 ± 2.0% in wild-type mice compared with 23 ± 5.0% in cftr−/− mice. For DHA, the values were 4.8 ± 1.1% and 2.4 ± 0.7%, respectively. In lung, AA was 9.5 ± 1.3% in wild-type mice compared with 21 ± 3.0% in cftr−/− mice. For DHA, the values were 6.4 ± 1.0% and 2.5 ± 1.4%, respectively. This demonstrates that the changes in AA and DHA observed in cftr−/− mice are not a consequence of differences in protein content.
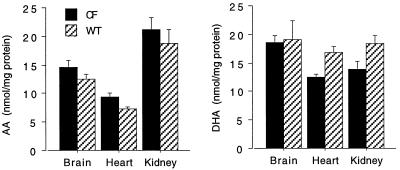
To determine whether the lipid imbalance observed in ileum, pancreas, and lung from cftr−/− mice was specific to organs clinically affected by CF, AA and DHA levels also were determined in brain, kidney, and heart, organs not clinically affected in CF. Although there was a trend toward an increase in phospholipid-bound AA and a decrease in phospholipid-bound DHA in brain and kidney homogenates from cftr−/− mice as compared with wild-type controls, these differences were not statistically significant (P > 0.05) (Fig. 2). In contrast, heart homogenates showed a significant increase in phospholipid-bound AA (P = 0.046) and a significant decrease in phospholipid-bound DHA (P = 0.011) in cftr−/− mice compared with wild-type controls (Fig. 2). This may be explained by the fact that heart, compared with kidney and brain, is known to contain a significant number of CFTR-regulated cells (19).
Figure 2.
AA and DHA levels from other organs from cftr−/− and wild-type mice. Homogenates from brain, heart, and kidney were prepared. AA and DHA were extracted from these organs from cftr−/− (CF) and wild-type (WT) mice. Results are expressed as the means ± S.D (n = 3). Only results from heart were statistically significant.
These results strongly suggest that the membrane lipid imbalance observed in cftr−/− mice may be related to loss of CFTR function. This association between CFTR and alterations in phospholipid-bound AA and DHA is supported by the fact that the pancreatic acini preparation is composed exclusively of CFTR-regulated cells (14, 20). Although the magnitude of this lipid imbalance may not relate directly to the severity of the phenotypic changes, ileum, the organ that shows the most severe pathologic changes in cftr−/− mice, displayed the greatest change in AA and DHA levels.
The Alterations in Phospholipid-Bound AA and DHA Levels Are Reversed with Oral Administration of DHA.
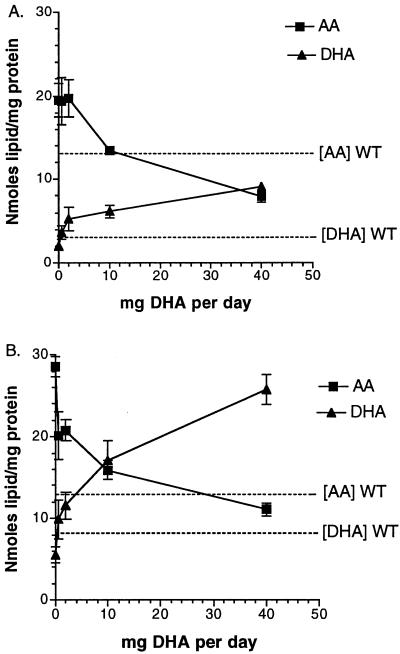
To test whether the increase in AA and decrease in DHA observed in cftr−/− mice are responsible for the expression of CF, DHA was administered orally to cftr−/− mice for 7 days at different concentrations, and AA and DHA levels subsequently were analyzed in pancreatic (Fig. 3A) and lung cell preparations (Fig. 3B) by GC/MS. Because cftr−/− mice need to be maintained on a liquid diet to prevent intestinal obstruction (18), wild-type and cftr−/− mice were fed with either Peptamen, a complete liquid enteral formulation composed principally of medium-chain triglycerides, essential fatty acids (0.16% linoleic and 0.023% α-linolenic acids), carbohydrates, and hydrolyzed protein or Peptamen supplemented with DHA as a stable emulsion. Because Peptamen contains as antioxidants 3 mg of vitamin E and 34 mg of vitamin C per 100 ml, this would minimize DHA oxidation during exposure of DHA/Peptamen emulsion to air during feeding. As confirmation, no DHA peroxides were detected by high-performance thin-layer chromatography–UV-reflectance spectrodensitometry. As shown in Fig. 3, oral administration of DHA produced a dose-dependent increase in DHA and a reciprocal decrease in AA concentration in pancreas and lung. At 10 and 40 mg/day, both AA and DHA levels were at or above the levels observed in wild-type mice, expressed as dotted lines in Fig. 3. Administration of DHA at 40 mg/day to cftr−/− mice resulted in a 4-fold decrease in membrane-bound AA and a 5-fold increase in membrane-bound DHA compared with cftr−/− mice fed with Peptamen alone.
Figure 3.
Effects of oral DHA administration on AA and DHA levels in preparations of pancreatic acini (A) and lung cells (B). Varying doses of DHA were administered orally for 7 days to cftr−/− mice, and AA and DHA levels were determined. The results are expressed as the mean nanomoles of lipid/mg protein ± SD as a function of mg of DHA administered orally per day (n = 3). For reference, mean AA and DHA levels from wild-type mice (WT) are indicated by dashed lines.
The Decrease in Phospholipid-Bound DHA Levels Is Not Caused by Defects in Intestinal Absorption or Hepatic Biosynthesis.
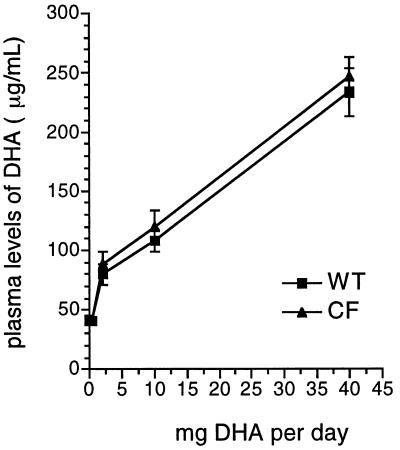
Endogenous plasma levels of DHA in cftr−/− and wild-type mice fed with Peptamen alone were not significantly different (P = 0.6) (Fig. 4), indicating that DHA biosynthesis in the liver, the principal source of DHA in plasma under these conditions (21), is not affected in cftr−/− mice. Also as shown in Fig. 4, DHA plasma levels in cftr−/− and wild-type mice after administration of increasing doses of DHA were not significantly different (P = 0.5), indicating normal intestinal absorption of DHA in cftr−/− mice.
Figure 4.
DHA plasma levels in wild-type (WT) and cftr−/− (CF) mice as a function of oral DHA dosing. Mice were fed varying doses of DHA for 7 days, and DHA levels were measured in plasma. Results are expressed as mean plasma level ± SD (n = 3).
Oral Administration of DHA Reverses Pancreatic and Ileal Pathology.
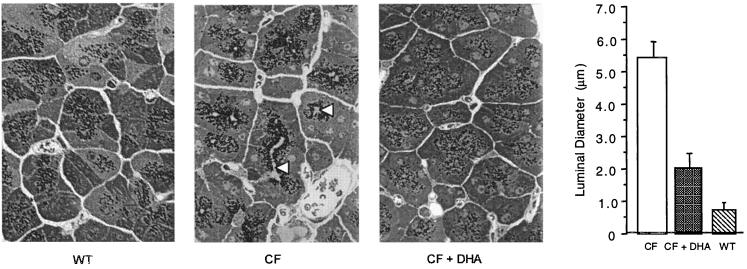
Fig. 5 shows pancreatic acinar cell morphology in wild-type, cftr−/− control, and cftr−/− mice fed 40 mg of DHA per day for 7 days. The cftr−/− control (Fig. 5, center micrograph) is characterized by massive luminal dilatation and zymogen granule accumulation at the apical pole of the acinar cells, two hallmark features of CF pathology (22). In sharp contrast, wild-type mice and cftr−/− mice fed DHA (Fig. 5, left and right micrographs, respectively) show no significant luminal dilatation, with zymogen granules more evenly dispersed within the cells. Morphometric analyses, shown at the far right in Fig. 5, indicated that luminal diameter was approximately 9-fold greater in cftr−/− mice compared with wild-type controls. Administration of 40 mg DHA/day to cftr−/− mice resulted in a 63% decrease in luminal diameter compared with untreated cftr−/− mice (P < 0.0001).
Figure 5.
Pancreatic morphology and morphometry from wild-type (WT) and cftr−/− (CF) mice, and cftr−/− mice treated orally with 40 mg per day of DHA for 7 days (CF + DHA). Mice were fed Peptamen or Peptamen containing 40 mg of DHA/day for 7 days. Tissue then was prepared for light microscopy. Representative sections (×200) are shown in the three micrographs. The arrowheads indicate the dilated duct lumen in cftr−/− mice. As shown at far right, pancreatic duct diameter was quantitated and expressed as mean diameter (μm) ± SD (n = 6).
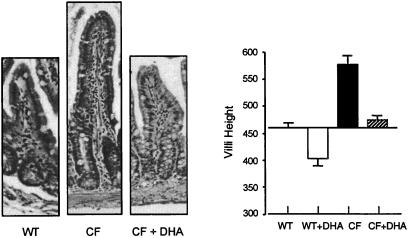
Ileal hypertrophy resulting from intestinal malabsorption is readily observed in cftr−/− mice (18), as shown in the micrographs of Fig. 6. Oral DHA decreased villus height to that observed in wild-type mice. Morphometric analyses are shown in Fig. 6 (Right). This confirms that oral DHA given to cftr−/− mice restores villus height to normal wild-type values. These effects of DHA were observed only when cftr−/− mice were fed 40 mg DHA per day and not with 10 mg or less (data not shown).
Figure 6.
Ileal morphology and morphometry from wild-type (WT) and cftr−/− (CF) mice and cftr−/− mice treated orally with 40 mg per day of DHA for 7 days (CF + DHA). Mice were fed Peptamen or Peptamen containing 40 mg of DHA/day (+DHA) for 7 days. Tissue then was prepared for light microscopy. Representative sections are shown (×200) in the three micrographs. (Right) Villous height was quantitated by using nih image software and expressed as arbitrary mean diameter units ± SD (n = 6).
Oral Administration of DHA Blocks Pseudomonas Endotoxin-Enhanced Lung Inflammation.
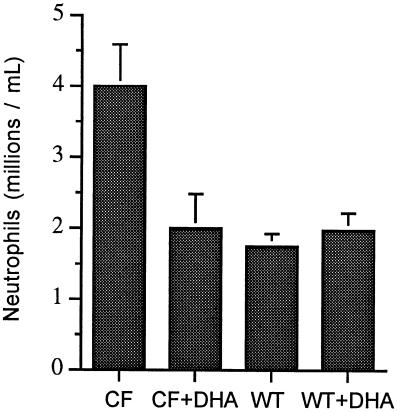
Although the lungs of UNC cftr−/− mice display minimal histologic changes, it is known that they are primed for inflammation. Therefore, to test the effects of DHA on the lungs of cftr−/− mice, we used a model of lung inflammation whereby wild-type and UNC cftr−/− mice were exposed to aerosolized Pseudomonas LPS daily for 3 days and neutrophil concentration in BAL fluid was determined. A significant increase in neutrophil concentration was observed in BAL fluid from cftr−/− mice compared with wild-type controls (Fig. 7). This enhancement in neutrophil infiltration was blocked selectively by pretreatment of cftr−/− mice with 40 mg DHA/day orally for 7 days. Pretreatment of wild-type mice with DHA had no effect on neutrophil concentration in BAL fluid after Pseudomonas LPS exposure.
Figure 7.
Neutrophil concentration in BAL fluid from wild-type (WT) and cftr−/− mice. Mice were fed Peptamen or Peptamen containing 40 mg of DHA/day (+DHA) for 7 days and then exposed to aerosolized Pseudomonas LPS daily for 3 days. Shown are the means ± SEM from at least five separate experiments. Statistically significant differences were observed in comparing CF with CF + DHA (P = 0.048), CF with WT (P = 0.0004), and CF with WT + DHA (P = 0.0107).
Reversal of Pancreatic Morphology Requires an Increase in Membrane-Bound DHA.
To determine whether the observed effects of DHA in cftr−/− mice are the result of a decrease in phospholipid-bound AA alone or require a concomitant increase in phospholipid-bound DHA, cftr−/− mice were fed 40 mg/day for 1 week of other n-3 fatty acids known to compete with AA and DHA biosynthesis and/or esterification into membrane phospholipids. Phospholipid-bound AA and DHA levels were analyzed and compared with Peptamen alone (control), as shown in Table 1. Ductal dilatation also was assessed by measurement of acinar lumen diameter in the pancreas. Administration of α-linolenic acid, a fatty acid known to compete with AA and DHA for the same elongation and desaturation enzymes, resulted in a 4-fold decrease in AA, a 2-fold further decrease in DHA, and a significant increase in ductal dilatation in pancreas. Administration of EPA, a fatty acid known to compete with AA for esterification at the sn-2 position of phospholipids, also resulted in a 4-fold decrease in AA but no significant changes in DHA levels or pancreas morphology. In sharp contrast, administration of DHA at the same concentration resulted in a 4-fold decrease in AA, a 5-fold increase in DHA, and reversal of ductal dilatation in the pancreas. These results strongly suggest that reversal of pancreatic morphology in cftr−/− mice requires an increase in phospholipid-bound DHA and that a decrease in phospholipid-bound AA alone is not sufficient to correct the histopathological manifestations of CF in pancreas from cftr−/− mice.
Table 1.
Phospholipid-bound AA and DHA levels and luminal diameter in pancreas from cftr−/− mice fed LNA, EPA, or DHA
| Fatty acid | AA | DHA | Luminal diameter |
|---|---|---|---|
| Control | 21.6 ± 7.6 | 1.5 ± 0.5 | 5.6 ± 1.4 |
| LNA | 5.5 ± 0.8 | 0.7 ± 0.07 | 7.3 ± 2.8 |
| EPA | 5.1 ± 0.3 | 2.2 ± 0.4 | 5.8 ± 1.2 |
| DHA | 4.9 ± 1.8 | 8.9 ± 1.5 | 1.8 ± 0.5 |
Values for AA and DHA are expressed in nmol/mg protein and for luminal diameter are expressed in μm and represent the mean ± SD from at least three separate experiments. LNA, α-linolenic acid.
Discussion
One of the significant findings emerging from this study is the identification of a profound membrane lipid imbalance in cftr−/− mice characterized by an increase in phospholipid-bound AA and a decrease in phospholipid-bound DHA. This was most pronounced in organs clinically affected by CF and represents the first disease where phospholipid-bound AA levels have been shown to be elevated. In addition, this fatty acid defect was selective, with the only other lipid alteration detected being an increase in 22:4n-6, a fatty acid intermediate downstream of AA.
Because AA and DHA compete with each other for the site of esterification at the sn-2 position of membrane phospholipids, two hypotheses could explain our observations: (i) the decrease in phospholipid-bound DHA observed in cftr−/− mice is the result of a primary defect in DHA biosynthesis, or (ii) the decrease in phospholipid-bound DHA is secondary to a primary increase in phospholipid-bound AA.
A primary defect in DHA biosynthesis could be explained by either an impairment in the enzymes involved in the elongation and desaturation of DHA precursors and/or by a defect in the translocation of DHA or its intermediates between different cell compartments. DHA biosynthesis requires multiple enzymatic steps as well as shuttling of DHA intermediates between different cell compartments including the endoplasmic reticulum and the peroxisomes. CFTR, in addition to its role in regulating chloride conductance in the apical membrane of epithelial cells, has been described as a multifunctional protein involved in the modulation of intracellular membrane trafficking (23). Whether a defect in CFTR directly interferes with DHA biosynthesis and/or is present in peroxisomes, where 24:6n-3 undergoes β-oxidation to generate DHA, remains to be elucidated. Because administration of 18:3n-3 resulted in a further decrease in DHA levels in cftr−/− but not in wild-type mice, this suggests that there may be a block in the biosynthesis of DHA. This is based on the fact that high concentrations of 18:3n-3 will monopolize the delta-6 desaturase enzyme responsible for the biosynthesis of 24:6n-3, the immediate precursor of DHA.
With regard to the second hypothesis, increased membrane-bound AA in cftr−/− mice could be explained by an up-regulation in AA biosynthesis from linoleic acid (18:2n-6). Alternatively, because AA and DHA compete with each other for the site of incorporation at the sn-2 position of phospholipids, the observed increase in AA also could be explained by either an increase in the rate of esterification of AA or a decrease in the rate of esterification of DHA into membrane phospholipids through changes in the affinities of acyl-CoA synthetase, acyl-CoA transferase, or phospholipase A2.
Fatty acids have been studied in CF patients, although analyses have been confined to examination of plasma and not tissues. These studies have led to the conclusion that a defect in fatty acid metabolism is present in CF patients independent of their nutritional status (24). This is characterized by an increase in plasma phospholipid palmitoleic acid and eicosatrienoic acid (20:3n-9) and a decrease in linoleic acid and DHA. Several hypotheses have been proposed to explain this abnormality including a decrease in fat intake (25), abnormal fatty acid turnover in membranes (26), and defects in desaturase activity (27). Protein–energy malnutrition cannot be invoked because the same abnormalities are observed in well-nourished CF patients compared with healthy controls (24). This suggests that the essential fatty acid deficiency observed in CF may be caused by a specific defect in fatty acid metabolism.
A defect in one of the enzymatic steps leading to DHA biosynthesis would explain why oral or parenteral dietary supplementation with precursor fatty acids only partially corrects the lipid defect in CF patients (28–30). This is corroborated by our data demonstrating that the administration of DHA, and not of precursor fatty acids, is required to correct the lipid abnormality as well as the pathologic changes observed in cftr−/− mice. Formulations containing DHA (e.g., fish oil) have been given to CF patients in an effort to down-regulate AA levels and decrease inflammation (31–33). No statistically significant clinical improvements were found in these studies between control and CF patients. However, DHA doses were significantly lower than the equivalent doses (based on body weight) used in our study to treat cftr−/− mice and would not be expected to significantly change phospholipid-bound AA and DHA levels. In addition, EPA, the major component of fish oil, would compete with DHA and, therefore, decrease its incorporation.
Alterations in levels of certain fatty acids may affect the lung response to infection. Animals placed on diets deficient in essential fatty acids, although not reproducing the CF phenotype, produce a bronchiolitis in which macrophage function and the ability to mount a lymphocytic response to Pseudomonas aeruginosa are impaired (34, 35). In addition, AA levels have been reported to be elevated in bronchial secretions of CF patients (5). Our data demonstrate that the enhancement in neutrophil infiltration in response to aerosolized Pseudomonas LPS can be selectively blocked by pretreatment of cftr−/− mice with oral DHA. That DHA therapy had no effect on neutrophil concentration in BAL fluid from wild-type mice indicates that DHA is correcting a specific biochemical defect in cftr−/− mice and not simply inhibiting mediators of inflammation.
Is the phenotypic expression of CF the result of an increase in phospholipid-bound AA, a decrease in phospholipid-bound DHA, or both? Suppression of phospholipid-bound AA levels without affecting phospholipid-bound DHA levels in pancreas had no observable effects on ductal dilatation in our cftr−/− mice. Further decreasing phospholipid-bound DHA worsened the phenotypic expression of CF in the pancreas. This strongly suggests that a decrease in phospholipid-bound DHA levels alone or in combination with an increase in phospholipid-bound AA may be responsible, at least in part, for the expression of CF in mouse pancreas.
In summary, our data would indicate that derangements in CFTR lead to abnormalities in fatty acid metabolism. That administration of DHA corrected the membrane lipid imbalance observed in the absence of a functional CFTR as well as reversed the phenotypic expression of CF in cftr−/− mice suggests that this membrane lipid imbalance plays a central role in the pathogenesis of CF. Because cftr−/− mice do not express the cAMP-regulated chloride channel (36), it cannot be ruled out that oral DHA administration, at these high doses, also may be exerting its effect through a CFTR-independent mechanism. This may include the activation and/or induction of other chloride conductance pathways (37), effects on other ion channels such as calcium (38, 39), sodium (40), and potassium (41, 42), the induction of apoptosis (43, 44), and the regulation of gene expression (45, 46).
One of the fundamental questions that remains to be answered is whether the membrane lipid imbalance observed in CFTR-regulated cells is representative of all membranes in these cells or of a specific subcellular domain. The DHA levels reported in this study represent total cellular content. However, DHA has been shown to preferentially accumulate in certain subcellular compartments (47). It is possible that a critical level of DHA in a specific membrane domain may be important in maintaining normal cell function in CFTR-regulated cells and that CFTR may be required to target DHA to these domains. In the absence of a functional CFTR, it may be necessary to increase DHA in all cell membranes to bring DHA concentration to the critical levels normally present in these specific domains. This is supported by the observation that reversal of the pathological manifestations of CF in cftr−/− mice required an overcorrection of total DHA levels. Another important question is how this membrane lipid imbalance impacts on cell function. It has been shown in animal models that fatty acid composition in membranes is important in the regulation of normal cell function (48). Because the mol percent of AA and DHA relative to total cell fatty acid content is altered significantly in cftr−/− mice compared with wild-type mice, it is likely that these changes may have an important impact on the physicochemical properties of membranes in these cells. However, the nature of this effect on membrane structure and function remains to be elucidated. Lastly, are these alterations in AA and DHA levels uniformly distributed in all phospholipids or are they found in certain phospholipids and not in others? The answers to some of these questions and the determination of the mechanism by which CFTR dysfunction leads to a decrease in DHA biosynthesis remain to be elucidated.
Acknowledgments
We thank Dr. Peter Durie from the Hospital for Sick Children in Toronto, Canada, and Drs. J. Thomas LaMont and Seth Alper from Beth Israel Deaconess Medical Center in Boston, MA, for their critical review of this manuscript. We also thank Joanne Cluette-Brown for her skilled assistance in the analysis of fatty acid methyl esters by GC/MS. This work was supported in part by the Beth Israel Deaconess Medical Center Ob/Gyn Foundation, the CF Foundation, and National Institutes of Health Grant R01 DK-52765 (S.D.F.).
Abbreviations
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- AA
arachidonic acid
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- BAL
bronchoalveolar lavage
- LPS
lipopolysaccharide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Collins F S. Science. 1992;256:774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 2.Riordan J R, Rommens J M, Kerem B S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J, et al. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Kerem B, Rommens J M, Buchanan J A, Markiewicz D, Cox T K, Chakravarti A, Buchwald M, Tsui L C. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 4.Li M, McCann J D, Anderson M P, Clancy J P, Liedtke C M, Nairn A C, Greengard P, Welsch M J. Science. 1989;244:1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- 5.Gilljam H, Strandvik B, Ellin A, Wiman L-G. Scand J Clin Lab Invest. 1986;46:511–518. doi: 10.3109/00365518609083706. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury N A, Jilling T, Berta G, Sorscher E J, Bridges R J, Kirk K L. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- 7.Scheele G A, Fukuoka S-I, Kern H F, Freedman S D. Pancreas. 1996;12:1–9. doi: 10.1097/00006676-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Lancet. 1983;2:257–258. [Google Scholar]
- 9.Heeckeren A, Walenga R, Konstan M W, Bonfield T, Davis P B, Ferkol T. J Clin Invest. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stillwell W, Jenski L J, Crump F T, Ehringer W. Lipids. 1997;32:497–506. doi: 10.1007/s11745-997-0064-6. [DOI] [PubMed] [Google Scholar]
- 11.Ehringer W, Belcher D, Wassall S R, Stillwell W. Chem Phys Lipids. 1990;54:79–88. doi: 10.1016/0009-3084(90)90063-w. [DOI] [PubMed] [Google Scholar]
- 12.Stillwell W, Ehringer W, Jenski L J. Lipids. 1993;28:103–108. doi: 10.1007/BF02535772. [DOI] [PubMed] [Google Scholar]
- 13.Kuehl F A, Egan R W. Science. 1980;210:978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- 14.Zeng W, Lee M G, Yan M, Diaz J, Benjamin I, Marino C, Kopito R, Freedman S, Cotton C, Muallem S, et al. Am J Physiol Cell Physiol. 1997;273:C442–C455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
- 15.Bruzzone R, Halban P A, Gjinovci A, Trimble E R. Biochem J. 1985;226:621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez J G, Storey B T. Mol Reprod Dev. 1995;42:334–346. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez J G, Touchstone J C. J Chromatogr. 1992;577:142–145. doi: 10.1016/0378-4347(92)80609-t. [DOI] [PubMed] [Google Scholar]
- 18.Kent G, Oliver M, Foskett J K, Frndova H, Durie P, Forstner J, Forstner G G, Riordan J R, Percy D, Buchwald M. Pediatr Res. 1996;40:233–241. doi: 10.1203/00006450-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 19.James A F, Tominaga T, Okada Y, Tominaga M. Circ Res. 1996;79:201–207. doi: 10.1161/01.res.79.2.201. [DOI] [PubMed] [Google Scholar]
- 20.Marino C, Matovick L M, Gorelick F S, Cohn J A. J Clin Invest. 1991;88:712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott B L, Bazan N G. Proc Natl Acad Sci USA. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebenthal E, Lerner A, Rolston D D K. In: The Pancreas: Biology, Pathobiology, and Diseases. Go V L W, DiMagno E P, Gardner J D, Lebenthal E, Reber H A, Scheele G A, editors. New York: Raven; 1993. pp. 1041–1082. [Google Scholar]
- 23.Jilling T, Kirk K L. Int Rev Cytol. 1997;172:193–241. doi: 10.1016/s0074-7696(08)62361-x. [DOI] [PubMed] [Google Scholar]
- 24.Roulet M, Frascarolo P, Rappaz I, Pilet M. Eur J Pediatr. 1997;156:952–956. doi: 10.1007/s004310050750. [DOI] [PubMed] [Google Scholar]
- 25.Farrell P M, Mischler E H, Engle M J, Brown D J, Lau S M. Pediatr Res. 1985;19:104–109. doi: 10.1203/00006450-198501000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Rogiers V, Dab I, Michotte Y. Pediatr Res. 1984;18:704–708. doi: 10.1203/00006450-198408000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd-Still J D, Johnson S B, Holman R T. Am J Clin Nutr. 1981;34:1–7. doi: 10.1093/ajcn/34.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Berg U, Kallner A, Kusoffsky E, Strandvik B. Monogr Pediatr. 1979;10:1–4. [PubMed] [Google Scholar]
- 29.Chase H P, Cotton E K, Elliot R B. Pediatrics. 1979;64:207–213. [PubMed] [Google Scholar]
- 30.Rosenlund M L, Selekman J A, Kim H K, Kritchevsky D. Pediatrics. 1977;59:428–432. [PubMed] [Google Scholar]
- 31.Katz D P, Manner T, Furst P, Askanazi J. Nutrition. 1996;12:334–339. doi: 10.1016/s0899-9007(96)80056-6. [DOI] [PubMed] [Google Scholar]
- 32.Kurlandsky L E, Bennink M R, Webb P M, Ulrich P J, Baer L J. Pediatr Pulmonol. 1994;18:24–27. doi: 10.1002/ppul.1950180404. [DOI] [PubMed] [Google Scholar]
- 33.Henderson W R, Jr, Astley S J, McCready M M, Kushmerick P, Casey S, Becker J W, Ramsey B W. J Pediatr. 1994;124:400–408. doi: 10.1016/s0022-3476(94)70362-0. [DOI] [PubMed] [Google Scholar]
- 34.Harper T B, Chase H P, Henson J, Henson P M. Am Rev Respir Dis. 1982;126:540–547. doi: 10.1164/arrd.1982.126.3.540. [DOI] [PubMed] [Google Scholar]
- 35.Craig-Schmidt M C, Faircloth S A, Teer P A, Weete J D, Wu C-Y. Am J Clin Nutr. 1986;44:816–824. doi: 10.1093/ajcn/44.6.816. [DOI] [PubMed] [Google Scholar]
- 36.Snouwaert J N, Brigman K K, Latour A M, Malouf N N, Boucher R C, Smithies O, Koller B H. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 37.Clarke L L, Brubb B R, Yankaskas J R, Cotton C U, McKenzie A, Boucher R C. Proc Natl Acad Sci USA. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Y F, Gomez A M, Morgan J P, Lederer W J, Leaf A. Proc Natl Acad Sci USA. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirafuji M, Ebihara T, Kawahara F, Nezu A, Taminura A, Saito H, Minami M. Res Commun Mol Pathol Pharmacol. 1998;102:29–42. [PubMed] [Google Scholar]
- 40.Kang J X, Leaf A. Proc Natl Acad Sci USA. 1996;93:3542–3546. doi: 10.1073/pnas.93.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poling S, Karanian J W, Salem N, Vicini S. Mol Pharmacol. 1995;47:381–390. [PubMed] [Google Scholar]
- 42.Poling J S, Vicini S, Rogawski M A, Salem N. Neuropharmacology. 1996;35:969–982. doi: 10.1016/0028-3908(96)00127-x. [DOI] [PubMed] [Google Scholar]
- 43.Calviello G, Palozza P, Piccioni E, Maggiano N, Frattucci A, Franceschelli P, Bartoli G M. Int J Cancer. 1998;75:699–705. doi: 10.1002/(sici)1097-0215(19980302)75:5<699::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 44.Fernandes G, Troyer D A, Jolly C A. Proc Nutr Soc. 1998;57:543–550. doi: 10.1079/pns19980080. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson J A, Gearing K, Widmark E, Tollet P, Stromstedt M, Berge R K, Gottlicher M. Prog Clin Biol Res. 1994;387:21–28. [PubMed] [Google Scholar]
- 46.Oshima M, Takahashi M, Oshima H, Tsutsumi M, Yazawa K, Sugimura T, Nishimura S, Wakabayashi K, Taketo M. Carcinogenesis. 1995;16:2605–2607. doi: 10.1093/carcin/16.11.2605. [DOI] [PubMed] [Google Scholar]
- 47.Croset M, Kinsella J E. Am Nutr Metab. 1989;33:125–142. doi: 10.1159/000177530. [DOI] [PubMed] [Google Scholar]
- 48.Spector A, Yorek M A. J Lipid Res. 1985;26:1015–1035. [PubMed] [Google Scholar]