Abstract
Nerve growth factor (NGF) is a neurotrophin with the ability to exert specific effects on cells of the immune system. Human monocytes/macrophages (M/M) infected in vitro with HIV type 1 (HIV-1) are able to produce substantial levels of NGF that are associated with enhanced expression of the high-affinity NGF receptor (p140 trkA) on the M/M surface. Treatment of HIV-infected human M/M with anti-NGF Ab blocking the biological activity of NGF leads to a marked decrease of the expression of p140 trkA high-affinity receptor, a concomitant increased expression of p75NTR low-affinity receptor for NGF, and the occurrence of apoptotic death of M/M. Taken together, these findings suggest a role for NGF as an autocrine survival factor that rescues human M/M from the cytopathic effect caused by HIV infection.
Cells of monocyte/macrophage (M/M) lineage play an important role in the pathogenesis and progression of the disease sustained by HIV (1–4). Their infection is characterized by a viral dynamic substantially different from that of lymphocytes (5). Indeed, in vivo HIV infection of activated CD4 lymphocytes accounts for the majority of the daily production of virus particles (productive infection) (6). At the same time, resting CD4 lymphocytes carrying the HIV genome are generally unable to sustain a complete and productive virus life cycle; for this reason, they minimally contribute to the daily virus production (latent infection) (7). M/M are productively infected by HIV as activated CD4 lymphocytes are, yet they survive HIV replication (3, 8, 9). Upon infection with a monocytotropic strain of HIV, M/M release ≥20,000 pg/ml of HIV p24 gag antigen and produce >108 copies of unspliced/multiply spliced RNA, starting at day 8–15, with a plateau lasting at least 60 days after infection (S.A., C.-F.P., R.C., and M.C.C., unpublished work). M/M are thus characterized by a stable production of high levels of virus particles, without major signs of HIV-induced cytopathic effect (persistent infection).
Persistently infected cells of M/M lineage represent a major reservoir of HIV in lymph nodes and extralymphoid tissues at all stages of the disease (10–14). In addition, they are the main target of HIV in the central nervous system, where their infection and consequent dysfunction are considered main pathogenetic events of the neuronal damage that often occur in the advanced stages of HIV infection (15–20).
The presence in the body of persistently infected M/M, as well as latently infected CD4 lymphocytes, represents a key challenge for therapeutic attempts to eradicate HIV infection by eliminating all cells harboring virus genome and/or sustaining virus replication for a long period of time. Despite various efforts, the characterization of the factor(s) allowing persistently infected M/M to overcome the cytopathic effect of HIV has been unsuccessful.
We concentrated our attention on nerve growth factor (NGF), a neurotrophic polypeptide necessary for the survival, development, and functions of peripheral and central neurons (21, 22). Other than its effect on neuronal system, NGF is able to regulate immune responses through direct and/or indirect effects on a number of immunocompetent cells (23–29). In addition, NGF potently affects several functions of M/M, such as differentiation, cytokine production, phagocytosis, and antimicrobial activity (30, 31). Finally, the ability of NGF to act as an autocrine survival factor for memory B lymphocytes is well recognized (32), as well as its capacity to promote the survival of mast cells (33).
Thus, for all of these reasons, we asked whether NGF may also play a role in the survival of macrophages upon HIV infection.
Materials and Methods
Virus.
The prototypic monocytotropic strain of HIV, named HIVBa-L (3), was used in all experiments. HIVBa-L was expanded and titrated in M/M. Virus stock contained 10,000 tissue culture 50% infective doses (TCID50) per ml.
Cells.
The procedure of M/M purification and culture is described in detail elsewhere (34). Briefly, human primary M/M were obtained from peripheral blood mononuclear cells (PBMC) of healthy seronegative donors. After PBMC were cultured for 5 days in plastic dishes, adherent cells were purified by repeated washings with warm medium and resulted in >95% M/M, as assessed by immunocytochemical staining and morphology. Adherent M/M were then cultured for an additional day (prior to virus challenge) in RPMI complete medium (GIBCO/BRL) supplemented with 20% endotoxin- and mycoplasma-free FCS (HyClone), penicillin and streptomycin (50 units/ml and 50 μg/ml, respectively), and 2 mM l-glutamine (complete medium). Unless otherwise specified, data presented in this paper were obtained from three different experiments each run in triplicate. A single donor provided M/M for each experiment.
Cell Infection and Virus Detection.
Virus challenge was performed by exposing M/M to 1,000 TCID50/ml of the monocytotropic strain HIV-1Ba-L, followed, after 4 hr, by extensive washing to remove virus excess. Virus production was periodically checked in the supernatants by a commercially available ELISA, which was able to detect HIV gag p24 (Abbott).
Antibody.
A neutralizing rabbit anti-NGF polyclonal Ab (32) was purified by affinity chromatography in agreement with a previously published method (35). A concentration of 40 μg/ml of such anti-NGF Ab, or of an IgG isotypic irrelevant Ab (where specified), was added to the cultures starting at 2 hr after infection and replaced at every medium change up to the end of the experiments.
NGF Production.
NGF production was assessed in the supernatants of M/M immediately before virus challenge and 5 days after HIV infection by an immunoassay, according to the method previously described (36) with minor modifications. Briefly, 96-well microtiter plates were coated with 50 μl of monoclonal mouse anti-NGF (0.02 mg/ml) diluted in 0.05 M carbonate buffer (pH 9.6) per well. To assess nonspecific binding, parallel wells were coated with equal amounts of purified mouse IgG. The optical density was measured at 575 nm with an ELISA reader (Dynatech), and the results were corrected by subtraction of background values.
Immunocytochemical analysis for NGF expression was performed on M/M at the same time point as NGF production assessment, which is immediately before virus challenge and 5 days after HIV infection. M/M were fixed with 4% paraformaldehyde dissolved in 0.1% phosphate buffer (pH 7.4), rinsed in PBS containing 0.2% Tween 20, and treated overnight with anti-NGF polyclonal Ab at 4°C. The primary Ab was then removed, and the cells were exposed to the secondary Ab, which was a biotinylated goat anti-rabbit Ig (IgG), and avidin-biotin-horseradish peroxidase (VECTASTAIN Elite ABC Kit; Vector Laboratories), followed by diaminobenzidine. Quantitative analysis of NGF-immunoreactive cells was carried out with a computerized image-analysis system (Zeiss Axiophot 2 microscope equipped with a Vidas Kontron system) (37).
Evalutation of Programmed Cell Death.
FACS analysis.
Infected or mock-infected M/M treated with purified polyclonal anti-NGF Ab were gently detached from plastic 5 days after virus challenge. Aliquots of 5 × 105 cells were centrifuged at 300 × g for 5 min; pellets were washed with PBS, placed on ice, and overlaid with 0.5 ml of a hypotonic fluorochrome solution containing 50 μg/ml propidium iodide, 0.1% sodium citrate, and 0.1% Triton X-100. After gentle resuspension in this solution, M/M were left at 4°C for 30 min, in the absence of light, before analysis. Propidium iodide-stained cells were analyzed with a FACScan Flow Cytometer (Becton Dickinson); fluorescence was measured between 565 and 605 nm. The data were acquired and analyzed by the Lysis II program (Becton Dickinson).
Immunocytochemical analysis.
M/M apoptotic nuclei were assessed by in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL), according to a previously described method. (38).
Morphological Analysis of NGF Receptors on the Cell Surface.
M/M were detached by gentle scraping and then stained with anti-p140 polyclonal Ab (Santa Cruz Biotechnology) or anti-p75 mAb generated by a hybridoma cell line from the American Type Culture Collection (Hb 8737), as previously described (39). Quantitative analysis of p140 trkA-immunoreactive cells was carried out on the number of p140 trkA- or p75NTR-immunopositive cells out of 100 cells per six microscopic fields at ×40 magnification using a computerized image-analysis system (Zeiss Axiophot 2 microscope equipped with a Vidas Kontron system).
Proapoptotic Proteins.
Aliquots of total protein extracts from different samples were resuspended in 0.1 M Tris⋅HCl buffer (pH 7.0) containing 1% SDS, 0.05% mercaptoethanol, 2.5% glycerol, and 0.001% bromophenol blue, and were boiled for 3 min and subsequently size fractionated by 7.5% SDS/PAGE. The gel was electroblotted overnight onto nitrocellulose paper at 40 mA, and the bands were revealed by horseradish peroxidase-conjugated goat anti-mouse or human IgG (Bio-Rad). The reaction was developed by the enhanced chemiluminescence detection system (Amersham Pharmacia). Molecular weights were evaluated by plotting Rf against log of molecular weight, using the Rainbow protein high molecular weight markers as a standard (Amersham Pharmacia).
Results
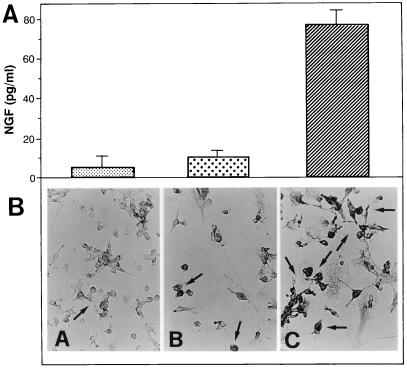
In the first set of experiments, we assessed whether M/M produce NGF upon HIV infection. As shown in Fig. 1A, uninfected M/M release a very limited amount of NGF; upon HIV infection, however, NGF production is increased about 8-fold (P < 0.0001, compared with uninfected M/M). Immunocytochemical studies confirm that control uninfected M/M show a marginal NGF immunoreactivity, whereas M/M infected with HIV are characterized by an intense NGF immunoreactivity (Fig. 1B) with a wide neurotrophin localization at the cytoplasmatic level.
Figure 1.
NGF production by macrophages infected with HIV. NGF production was assessed in the supernatants of M/M immediately before virus challenge (A, small dots) and 5 days after mock infection (big dots) or HIV infection (hatched bar). Immunocytochemical analysis was performed on M/M (B) at the same time points, that is, immediately before virus challenge (A) and 5 days after mock infection (B) or HIV infection (C). NGF production in the supernatants of HIV-infected M/M is statistically greater than that found in the supernatants of mock-infected M/M (either before or after mock infection) [ANOVA: F(2, 6) = 96.448; P = 0.001]. Values represent mean ± SE of an experiment representative of three.
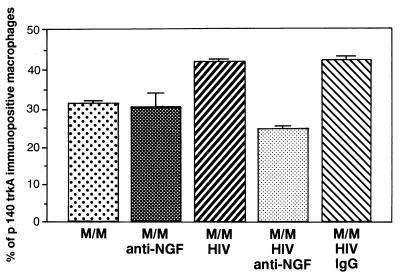
We next asked whether HIV infection somewhat modifies the expression of the two types of receptors for NGF in M/M. High-affinity NGF-trkA receptor (p140 trkA) is a 140-kDa molecule with a transmembrane tyrosine kinase domain that is encoded by the trk protooncogene and characterized by a high-affinity (Kd = 20 pM) for the ligand (40–42). Our immunocytochemical studies demonstrate that p140 trkA receptor expression is increased by about 30% in HIV-infected M/M, compared with uninfected cells. This effect is related to the HIV-1-mediated overproduction of NGF because the treatment with a neutralizing Ab against NGF markedly decreases p140 trkA immunoreactivity only in HIV-infected M/M (but not in uninfected ones) (Fig. 2). This suggests that the overexpression of p140 trkA receptor by infected M/M occurs only in the presence of both virus infection and consequent production of endogenous NGF.
Figure 2.
Expression of high-affinity receptors on macrophages infected by HIV. Treatment of HIV-infected M/M with anti-NGF Ab (M/M + HIV + anti-NGF), but not with an IgG isotypic Ab (M/M + HIV + IgG), modifies the cellular expression of p140 trkA high-affinity receptor. Quantitative analysis of p140 trkA-immunoreactive cells carried out with a computerized image-analysis system (Zeiss Axiophot 2 microscope equipped with a Vidas Kontron system) shows that the increase of p140 trkA immunopositive cells in HIV-infected human M/M (M/M + HIV) was statistically significant (ANOVA: F(4, 85) = 116.017; P < 0.01) compared with mock-infected human M/M (M/M). Treatment of HIV-infected M/M with anti-NGF Ab (M/M + HIV + anti-NGF) yielded a statistically significant decrease (P < 0.01) of p140 trkA immunoreactivity in comparison to HIV-infected M/M (M/M + HIV), mock-infected human M/M (M/M + anti-NGF), or HIV-infected M/M with an irrelevant IgG Ab (M/M + HIV + IgG).
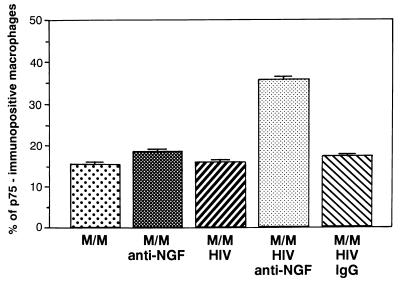
The next step of our study was to assess the expression of the low-affinity NGF-p75NTR receptor (p75NTR). On one hand, this membrane receptor is up-modulated in the case of limited concentrations of NGF. At the same time, however, the overexpression of p75NTR under the same conditions is able to trigger apoptosis in primary neurons and in neuronal cell lines (43, 44). Immunocytochemical analysis (Fig. 3) shows that p75NTR receptor is expressed by about 15% of control uninfected M/M (either treated or not treated with anti-NGF Ab), as well as of untreated M/M infected by HIV. However, the treatment with the neutralizing anti-NGF Ab (but not with an IgG isotypic irrelevant Ab) increases the number of HIV-infected M/M expressing p75NTR receptors on their surface to 35%. Thus, HIV infection and NGF starvation (by treatment with a neutralizing Ab) are both required to enhance the expression of p75NTR receptor on the M/M surface.
Figure 3.
Expression of low-affinity receptors in macrophages infected with HIV. A dramatic increase of p75NTR receptor expression was consistently obtained by treating HIV-infected M/M with anti-NGF Ab. An IgG isotypic Ab was totally ineffective. A quantitative analysis of p75NTR immunoreactive cells (carried out with a computerized image-analysis system) shows a statistically significant increase of p75NTR immunopositive cells in HIV-infected human M/M treated with anti-NGF Ab compared with all other samples, either infected or not (ANOVA: F(4, 85) = 289.354; P = 0.001).
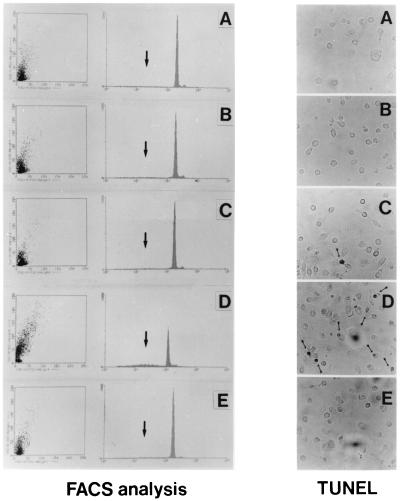
On the basis of these results, we then assessed whether the depletion of NGF mediated by a neutralizing Ab, and the consequent overexpression of p75NTR receptor, trigger apoptosis in HIV-infected M/M. To do so, extensive FACS analysis and immunocytochemical studies were performed. Results obtained by propidium iodide staining (Fig. 4 Left ) indicate that signs of programmed cell death are detectable in <10% of uninfected M/M, either treated or not treated with anti-NGF Ab. Similar results were also obtained in HIV-infected but nontreated M/M. This latter result is not surprising in view of the fact that M/M are resting cells able to remain in culture for weeks or months without further stimuli, even if infected by HIV. However, the treatment of HIV-infected M/M with the anti-NGF neutralizing Ab induces massive signs of apoptosis in about 45% (range 34–58% in different experiments) of cultured M/M (Fig. 4 Left).
Figure 4.
Programmed cell death in HIV-infected macrophages exposed to anti-NGF Ab. (Left) FACS analysis. Apoptosis was detected by DNA labeling with propidium iodide, a fluorescent intercalating dye that allows DNA quantification. Apoptotic nuclei appeared as a broad hypodiploid DNA peak (black arrow in each panel) easily discriminable from the narrow peak of nuclei with normal diploid DNA counted in the red fluorescence channel. DNA fragmentation has been detected in 5% of mock-infected human M/M (A). Results in the same range were observed in HIV-infected M/M (9.1% of propidium-positive cells; B), in mock-infected M/M exposed to anti-NGF Ab (6.9%; C), or in HIV-infected M/M treated with the IgG isotypic Ab (8.7%; E). By contrast, exposure of HIV-infected cells to anti-NGF Ab (D) induces DNA fragmentation in 40.3% of M/M in this experiment representative of five different tests. (Right) TUNEL. Immunocytochemical studies performed by TUNEL show nuclei with round condensed chromatin in HIV-infected M/M exposed to anti-NGF Ab (D) far more than in any of the other M/M samples tested (A, B, C, and E). The figure presents data from a typical experiment of three.
A time-course analysis performed by double staining with propidium iodide and annexin V shows that a peak of apoptotic cells can be detected by both methods at a single time point, ranging, in different experiments, from day 5 to day 8 after virus challenge and treatment with anti-NGF neutralizing Ab. By contrast, apoptosis in HIV-infected anti-NGF-treated M/M at time points other than the day of the peak were superimposable to the background levels detected in all other M/M samples analyzed (either infected or not infected) (data not shown). This result suggests that the apoptotic event occurs at the same time in all susceptible M/M infected by HIV. This is in line with results obtained in another model, showing that apoptosis of PC12 cells occurs rapidly and at the same time in all susceptible cells upon NGF withdrawal (45).
Additional evidence that exposure to neutralizing anti-NGF Ab mediates an apoptotic signal in HIV-infected M/M was provided by TUNEL. Indeed, chromatin degradation occurs in HIV-infected M/M treated with anti-NGF Ab 4- to 5-fold more than in infected M/M treated with an irrelevant isotypic IgG Ab (or in uninfected M/M exposed to anti-NGF) (Fig. 4 Right).
To further characterize the apoptosis elicited by the NGF withdrawal in HIV-infected M/M, we have also investigated the expression of a panel of genes whose induction has been shown to take place in the dying cells of HIV-infected individuals (46, 47). We found that two proapoptotic genes, such as Bax and “tissue” transglutaminase (tTG), are constitutively expressed in HIV-infected M/M. By contrast, the levels of both CD95L (Fas ligand) and its receptor CD95, although constitutively expressed by M/M, were even decreased in HIV-infected M/M undergoing apoptosis after NGF deprivation (data not shown).
Immunofluorescence analysis shows that the number of primary M/M expressing HIV antigens under these conditions does not exceed 50% of cultured cells at any time point tested (data not shown) and is dramatically decreased by treatment with anti-NGF Ab. Thus, the rate of infected M/M undergoing apoptosis after treatment with anti-NGF Ab (Fig. 4) is in the range of that showing productive infection by HIV.
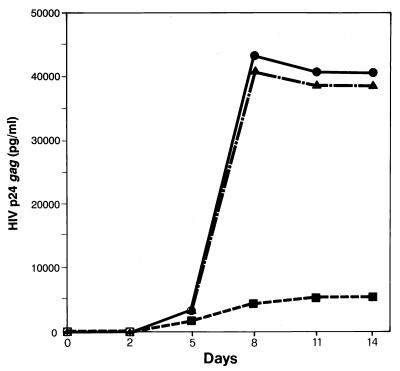
The direct consequence of this phenomenon could be a decrease of virus production from cultures of HIV-infected, anti-NGF-treated M/M. Indeed, a time-course analysis shows that M/M cultures exposed to anti-NGF Ab produce at least 8- to 10-fold less HIV p24 gag antigen than untreated HIV-infected M/M (Fig. 5). This starts at day 5 after infection, shows a maximal difference at day 8 (the time point that follows the peak of apoptosis found in this experiment in anti-NGF-treated HIV-infected M/M), and plateaus thereafter. This finding confirms that cultures of M/M infected by HIV are subjected to programmed cell death by NGF starvation and, thus, to a consequent decrease of virus production.
Figure 5.
Effect of NGF starvation upon HIV production in macrophages. A dramatic difference of virus production was detected between HIV-infected M/M exposed to anti-NGF Ab (■), HIV-infected M/M (●), or HIV-infected M/M exposed to an IgG-isotypic irrelevant Ab (▴). HIV p24 gag antigen production was evaluated by commercially available ELISA at days 2, 5, 8, 11, and 14 after virus infection. The figure represents a typical experiment of three.
Discussion
Overall results indicate that NGF is an autocrine survival factor for M/M infected by HIV, that is implicated in the resistance to the cytopathic effect normally induced by this virus in other cells.
Previous studies supported a role of NGF in the modulation of virus replication and neuronal damage mediated by virus proteins. NGF enhances HIV gene expression and replication in neuronal and glial cell lines by means of cellular differentiation (48). gp120, the HIV envelope glycoprotein, is able to affect NGF and NGF receptors in adult rat brain (49). Furthermore, NGF is implicated in maintaining latency and in delaying the cytopathic effect of herpes simplex virus in neurons (50–52). At the same time, the critical role of autocrine NGF in the survival of memory B lymphocytes (cells of ontogenetic origin similar to that of monocytes) has been clearly shown (32), as well as the effect of NGF upon growth and differentiation of bone marrow stem cells (53). Overall published data thus suggest that NGF supports differentiation and survival of various nonneuronal cell types, affects virus replication, and interacts with HIV proteins at different cellular and organ levels. This may help in explaining our finding about its role in the regulation of the survival of M/M infected by HIV.
A question raised by our observations is regarding the mechanism(s) by which NGF acts as a rescue factor in HIV-infected M/M. One possibility could be the release of biologically active compounds influenced by the neurotrophin, such as tumor necrosis factor (TNF)-α and IL-1β, whose production can be influenced by NGF (54, 55); nevertheless, there is no evidence in the literature that treatment with Abs neutralizing such cytokines induces apoptosis in HIV-infected primary M/M. Even more, NGF protects PC12 cells, a suitable model for NGF studies, from the proapoptotic effect of TNF-α (56). Finally, although HIV infection of M/M induces the production of various chemokines and cytokines, neutralization of these factors (either by Abs or by specific inhibitors) has never been shown to trigger apoptosis in M/M. Further studies, beyond the scope of this paper, may be needed to clarify the fine interplays between cytokines and HIV in M/M. Nevertheless, from the data available, there is no evidence that the antiapoptotic effect of NGF on HIV-infected M/M is directly mediated by these factors.
Another possibility that may explain the effect of NGF on HIV-infected M/M is that such a neurotrophic factor acts on some specific events associated with the apoptotic mechanism. NGF-trkA interaction, for example, modulates several functional properties of monocytes, such as triggering the respiratory burst activity (29). Similarly, it has been demonstrated that NGF prevents apoptosis in rat peritoneal mast cells through the p140 trkA tyrosine kinase receptor (57). Other than through interaction with p140 trkA, recent findings demonstrate that NGF can also act by means of the p75NTR receptor to modulate apoptosis. Indeed, p75NTR-dependent apoptosis dramatically depends on whether the receptor is coexpressed with p140 trkA and on NGF supply. In fact, when p75NTR is coexpressed with p140 trkA in the presence of NGF, it enhances NGF binding to the receptor and stimulates cell survival (58). By contrast, expression of p75NTR induces apoptosis if NGF is removed or blocked by a neutralizing Ab (59–61).
HIV-infected M/M fit this scenario. In fact, we demonstrate here that HIV-infected M/M (but not control uninfected M/M) actively produce NGF and express enhanced levels of p140 trkA. Under these conditions, M/M can survive in culture for 60 days, even in the case of productive infection by HIV. Conversely, the expression of p140 trkA and the survival of HIV-infected M/M are dramatically decreased when endogenous NGF is depleted from the medium by a neutralizing Ab, and p75NTR is markedly enhanced. Thus, the expression of p75NTR plays a role as a mediator of a signaling pathway acting upon survival/death of HIV-infected M/M, depending of the presence or absence of NGF in the culture. This event is not surprising, in view of the analogy of this receptor with TNFRI, TNFRII, Fas-ApoI, and CD40, all membrane receptors involved in the regulation of cell survival (62).
The p75NTR-mediated signaling cascade leading to apoptosis has been deeply studied, but not fully defined (59–61). In our case, HIV-infected M/M constitutively express many proapoptotic genes, such as Bax and tTG, which have been shown to be involved in apoptosis in HIV-infected individuals (46, 47). At the same time, the levels of CD95/CD95L proteins drop upon the neurotrophin withdrawal, thus suggesting that the apoptotic cascade modulated by NGF by means of the p75NTR is not mediated by this pathway, in line with recent findings in HIV-infected individuals (63). Further work is thus required to clarify the fine intracellular pathway(s) involved in the apoptotic cascade in HIV-infected M/M.
The results reported in this paper have substantial implications in the pathogenesis of HIV infection. M/M in spleen, lymph nodes, bone marrow, liver, and other tissues may take advantage of their autocrine NGF (and of that produced by other cells), survive for a very long period of time, and continuously produce virus particles. This might be particularly relevant at the level of the central nervous system, where M/M represent the majority of cells infected by HIV, and where most of the resident cells are able to produce NGF. Nothing is known regarding the survival of macrophages (either infected or not) in the brain of patients. A recent report suggests that HIV-infected monocytes injected in the brain of severe combined immunodeficient (SCID) mice are able to move, survive for several months, continue to produce HIV particles, and sustain irreversible neurological damage (64). Thus, a very long survival of HIV-infected M/M in the central nervous system is quite conceivable.
Overall data may have potential implications for the therapy of HIV infection. The elimination of all cells bearing HIV genome (either latently or persistently infected by the virus) is a key factor for therapeutic attempts aimed at the eradication of the virus from the body. For this purpose, the recognition of NGF and other factors allowing chronically infected cells to survive HIV infection may open the way to new therapeutic strategies aimed at causing selective death of infected cells. The present finding establishes an important function of NGF in viral infection and suggests that new and innovative research efforts in this field may provide additional useful information for the understanding of the mechanisms implicated in this effect.
Acknowledgments
We thank Mrs. Tania Guenci and Fabio Marcuccilli for technical help. This work was supported by grants from AIDS Project of the Istituto Superiore di Sanità (ISS), Biomed Project of European Economic Community, National Research Council of Italy (P.F. Biotechnology 5), “Ricerca Corrente” Istituto Ricovero e Cura a Carattere Scientifico L. Spallanzani, and Government Office of University and Scientific Research (Rome, Italy). A.A. was supported by an “AIDS” ISS grant.
Abbreviations
- M/M
monocyte/macrophage
- NGF
nerve growth factor
- TCID50
tissue culture 50% infective dose
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP nick-end labeling
References
- 1.Meltzer M S, Nakamura M, Hansen B D, Turpin J A, Kalter D C, Gendelman H E. AIDS Res Hum Retroviruses. 1990;6:967–971. doi: 10.1089/aid.1990.6.967. [DOI] [PubMed] [Google Scholar]
- 2.Popovic M, Gartner S. Lancet. 1987;ii:916. doi: 10.1016/s0140-6736(87)91403-6. [DOI] [PubMed] [Google Scholar]
- 3.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 4.Ho D D, Rota T R, Hirsch M S. J Clin Invest. 1986;77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagnarelli P, Valenza A, Menzo S, Sampaolesi R, Varaldo P E, Butini L, Montroni M, Perno C F, Aquaro S, Mathez D, et al. J Virol. 1996;70:7603–7613. doi: 10.1128/jvi.70.11.7603-7613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 7.Chun T W, Carruth L, Finzi D, Shen Y, Di Giuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, et al. Nature (London) 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 8.Perno C F, Newcomb F M, Davis D A, Aquaro S, Humphrey R W, Caliò R, Yarchoan R. J Infect Dis. 1998;178:413–422. doi: 10.1086/515642. [DOI] [PubMed] [Google Scholar]
- 9.Aquaro S, Caliò R, Balestra E, Bagnarelli P, Cenci A, Bertoli A, Tavazzi B, Di Pierro D, Francesconi M, Abdelahad D, et al. J Biol Regul Homeostatic Agents. 1998;12:23–27. [PubMed] [Google Scholar]
- 10.Armstrong J A, Horne R. Lancet. 1984;ii:370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- 11.Tschachler E, Groh V, Popovic M, Mann D L, Konrad K, Safai B, Eron L, diMarzo Veronese F, Wolff K, Stingl G. J Invest Dermatol. 1987;88:233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- 12.McElrath M J, Pruett J E, Cohn Z A. Proc Natl Acad Sci USA. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattern C F, Murray K, Jensen A, Farzadegan H, Pang J, Modlin J F. Pediatrics. 1992;89:207–209. [PubMed] [Google Scholar]
- 14.Orenstein J M, Fox C, Wahl S M. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 15.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 16.Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, et al. Am J Pathol. 1987;126:403–410. [PMC free article] [PubMed] [Google Scholar]
- 17.Gabuzda D H, Ho D D, de la Monte S M, Hirsch M S, Rota T R, Sobel R A. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- 18.Giulian D, Vaca K, Noonan C A. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 19.Tyor W R, Power C, Gendelman H E, Markham R B. Proc Natl Acad Sci USA. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton S A, Gendelman H E. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 21.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 22.Levi-Montalcini R, Aloe L, Alleva E. Prog Neuroendocrinimmunol. 1990;3:1–10. [Google Scholar]
- 23.Gee A P, Boyle M D, Munger K L, Lawman M J, Young M. Proc Natl Acad Sci USA. 1983;80:7215–7218. doi: 10.1073/pnas.80.23.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce F L, Thompson H L. J Physiol (London) 1986;372:379–393. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorpe L W, Perez-Polo J R. J Neurosci Res. 1987;18:134–139. doi: 10.1002/jnr.490180120. [DOI] [PubMed] [Google Scholar]
- 26.Labouyrie E, Parrens M, de Mascarel A, Bloch B, Merlio J P. J Neuroimmunol. 1997;77:161–173. doi: 10.1016/s0165-5728(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Auffray I, Chevalier S, Froger J, Izac B, Vainchenker B, Gascan H, Coulombel L. Blood. 1996;88:1608–1618. [PubMed] [Google Scholar]
- 28.Aloe L, Bracci-Laudiero L, Bonini S, Manni L. Allergy. 1997;52:883–894. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 29.Kannan Y, Usami K, Okada M, Shimizu S, Matsuda H. Biochem Biophys Res Commun. 1992;186:1050–1056. doi: 10.1016/0006-291x(92)90853-d. [DOI] [PubMed] [Google Scholar]
- 30.Ehrhard P B, Ganter U, Stalder A, Bauer J, Otten U. Proc Natl Acad Sci USA. 1993;90:5423–5427. doi: 10.1073/pnas.90.12.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Susaki Y, Shimizu S, Katakura K, Watanabe N, Kawamoto K, Matsumoto M, Tsudzuki M, Furusaka T, Kitamura Y, Matsuda H. Blood. 1996;88:4630–4637. [PubMed] [Google Scholar]
- 32.Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni N, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 33.Aloe L, Levi-Montalcini R. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 34.Perno C F, Yarchoan R. Current Protocols in Immunology. New York: Greene & Wiley; 1993. pp. 12.4.1–12.4.11. [Google Scholar]
- 35.Stoeckel K, Gagnon C, Guroff G, Thoenen H. J Neurochem. 1976;26:1207–1211. doi: 10.1111/j.1471-4159.1976.tb07008.x. [DOI] [PubMed] [Google Scholar]
- 36.Bracci L, Aloe L, Levi-Montalcini R, Buttinelli C, Shilter D, Gillessen S, Otten U. Neurosci Lett. 1992;147:9–12. doi: 10.1016/0304-3940(92)90762-v. [DOI] [PubMed] [Google Scholar]
- 37.Hazen-Martin D J, Simson J A J. Histochem Cytochem. 1984;32:30–36. doi: 10.1177/32.1.6361117. [DOI] [PubMed] [Google Scholar]
- 38.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambiase A, Bracci-Laudiero L, Bonini S, Starace G, D’Elios M M, De Carli M, Aloe L. J Allergy Clin Immunol. 1997;100:408–414. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- 40.Sutter A, Riopelle R J, Harris-Warrick R M, Shooter E M. J Biol Chem. 1979;254:5972–5982. [PubMed] [Google Scholar]
- 41.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan D R, Hempstead B L, Martin-Zanca D, Chao M V, Parada L F. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 43.Lee K F, Davies A M, Jaenisch R. Development (Cambridge, UK) 1994;120:1027–1033. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- 44.Chao M V, Hempstead B L. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 45.Rong P, Bennie A M, Epa W R, Barrrett G L. J Neurochem. 1999;72:2294–2300. doi: 10.1046/j.1471-4159.1999.0722294.x. [DOI] [PubMed] [Google Scholar]
- 46.Gougeon M L. Psychoneuroendocrinology. 1997;22:33–39. doi: 10.1016/s0306-4530(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 47.Amendola A, Gougeon M L, Poccia F, Bondurand A, Fesus L, Piacentini M. Proc Natl Acad Sci USA. 1996;93:11057–11062. doi: 10.1073/pnas.93.20.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ensoli F, Ensoli B, Thiele C J. Virology. 1994;200:668–676. doi: 10.1006/viro.1994.1230. [DOI] [PubMed] [Google Scholar]
- 49.Bagetta G, Corasaniti M T, Aloe L, Berliocchi L, Costa N, Finazzi-Agro A, Nistico G. Proc Natl Acad Sci USA. 1996;93:928–933. doi: 10.1073/pnas.93.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilcox C L, Johnson E M J. J Virol. 1987;61:2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilcox C L, Johnson E M J. J Virol. 1988;62:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aloe L. Int J Dev Neurosci. 1987;5:357–366. doi: 10.1016/0736-5748(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 53.Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, Kitamura Y. J Exp Med. 1991;174:7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patterson J C, Childs G V. Endocrinology. 1994;135:1697–1704. doi: 10.1210/endo.135.4.7925134. [DOI] [PubMed] [Google Scholar]
- 55.Woolf C J, Allchorne A, Safieh-Garabedian B, Poole S. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heviv R, Stein R. J Neurosci Res. 1999;55:269–277. doi: 10.1002/(SICI)1097-4547(19990201)55:3<269::AID-JNR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 57.Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Blood. 1995;86:4638–4644. [PubMed] [Google Scholar]
- 58.Casaccia-Bonnefil P, Kong H, Chao M V. Cell Death Differ. 1998;5:357–364. doi: 10.1038/sj.cdd.4400377. [DOI] [PubMed] [Google Scholar]
- 59.Bredesen D E, Ye X, Tasinato A, Sperandio S, Wang J J L, Assa-Munt N, Rabizadeh S. Cell Death Differ. 1998;5:365–371. doi: 10.1038/sj.cdd.4400378. [DOI] [PubMed] [Google Scholar]
- 60.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 61.Soilu-Hanninen M, Ekert P, Bucci T, Syroid D, Bartlett P F, Kilpatrick T J. J Neurosci. 1999;19:4828–4838. doi: 10.1523/JNEUROSCI.19-12-04828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raffioni S, Bradshaw R A, Buxser S E. Annu Rev Biochem. 1993;62:823–850. doi: 10.1146/annurev.bi.62.070193.004135. [DOI] [PubMed] [Google Scholar]
- 63.Sieg S, Smith D, Yildirim Z, Kaplan D. Proc Natl Acad Sci USA. 1997;94:5860–5865. doi: 10.1073/pnas.94.11.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman H E. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]