Abstract
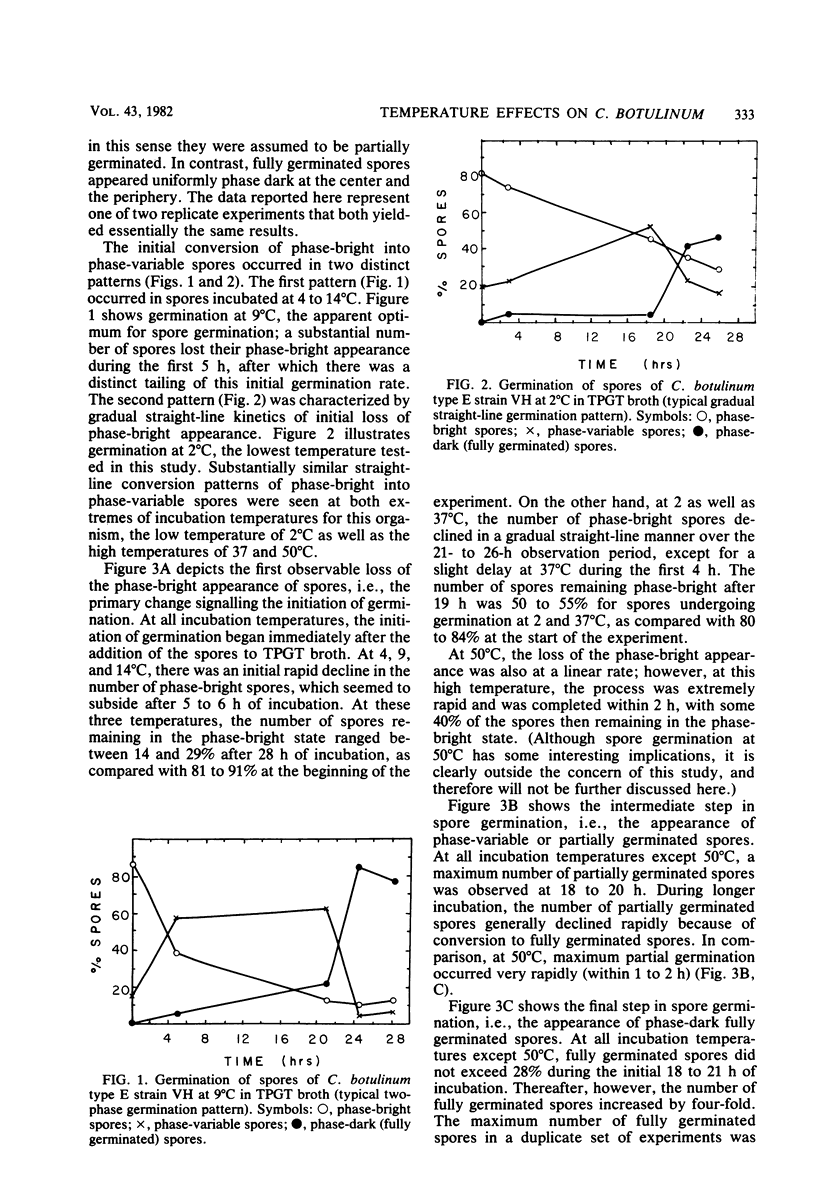
Spore germination and vegetative growth of Clostridium botulinum type E strain VH at 2 to 50 degrees C were studied. At all of these temperatures, germination began immediately after the addition of the spores to the germination medium. Microscopic observations during germination revealed three types of spores: phase bright (ungerminated), phase variable (partially germinated), and phase dark (fully germinated). At all temperatures except 50 degrees C, there was a pronounced lag between the initial appearance of phase-variable spores and their eventual conversion to phase-dark spores. The number of partially germinated spores increased steadily, reaching 40 to 60% by 18 to 21 h of incubation. During this time, phase-dark, fully germinated spores developed slowly and did not exceed 28% in any of the samples. At 18 to 26 h of incubation, the rate of full germination increased abruptly four-fold. There was extensive and relatively rapid germination at 2 degrees C, the lowest temperature tested, yielding about 60% phase-variable spores by 18 h, which became phase-dark by 26 h of incubation. The optimum temperature for partial and full germination was consistently 9 degrees C. Germination at 50 degrees C was exceptionally rapid and was completed within 1 to 2 h, although 40% remained phase bright. Vegetative cells showed detectable growth at 6 to 41 degrees C, with a distinct optimum at 32.5 degrees C. No growth occurred at 50 degrees C, and only marginal growth was observed at 6 to 14 degrees C. The psychrophilic nature of the germination process coupled with the cold tolerance of vegetative growth appears to give C. botulinum type E an advantage in cold climates as well as in cold-stored foods.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cann D. C., Wilson B. B., Hobbs G., Shewan J. M., Johannsen A. The incidence of Clostridium botulinum type E in fish and bottom deposits in the North Sea and off the coast of Scandinavia. J Appl Bacteriol. 1965 Dec;28(3):426–430. doi: 10.1111/j.1365-2672.1965.tb02173.x. [DOI] [PubMed] [Google Scholar]
- DOLMAN C. E., CHANG H., KERR D. E., SHEARER A. R. Fish-borne and type E botulism: two cases due to home-pickled herring. Can J Public Health. 1950 Jun;41(6):215–229. [PubMed] [Google Scholar]
- Duncan C. L., Foster E. M. Effect of sodium nitrite, sodium chloride , and sodium nitrate on germination and outgrowth of anaerobic spores. Appl Microbiol. 1968 Feb;16(2):406–411. doi: 10.1128/am.16.2.406-411.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Foster E. M. Nitrite-induced germination of putefactive anaerobe 3679h spores. Appl Microbiol. 1968 Feb;16(2):412–416. doi: 10.1128/am.16.2.412-416.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss H. H. Distribution of Clostridium botulinum. Appl Environ Microbiol. 1980 Apr;39(4):764–769. doi: 10.1128/aem.39.4.764-769.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


