Abstract
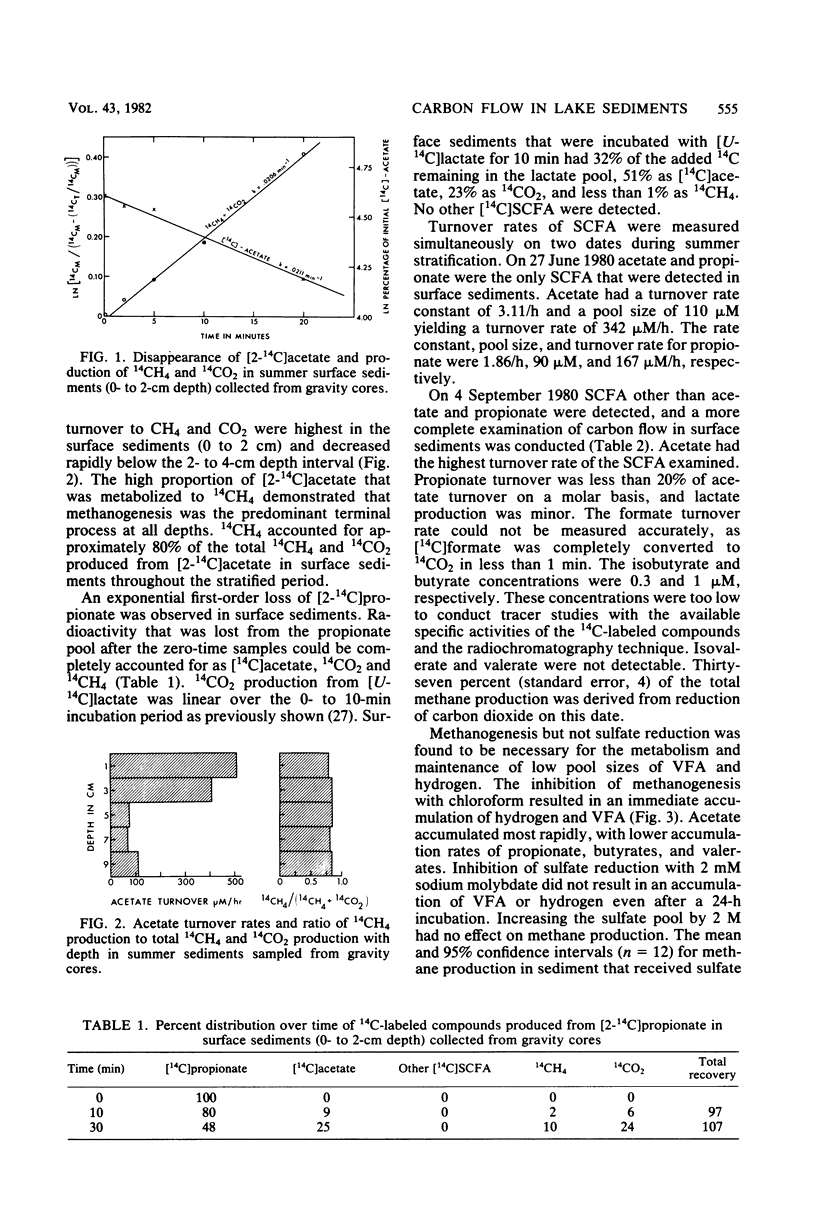
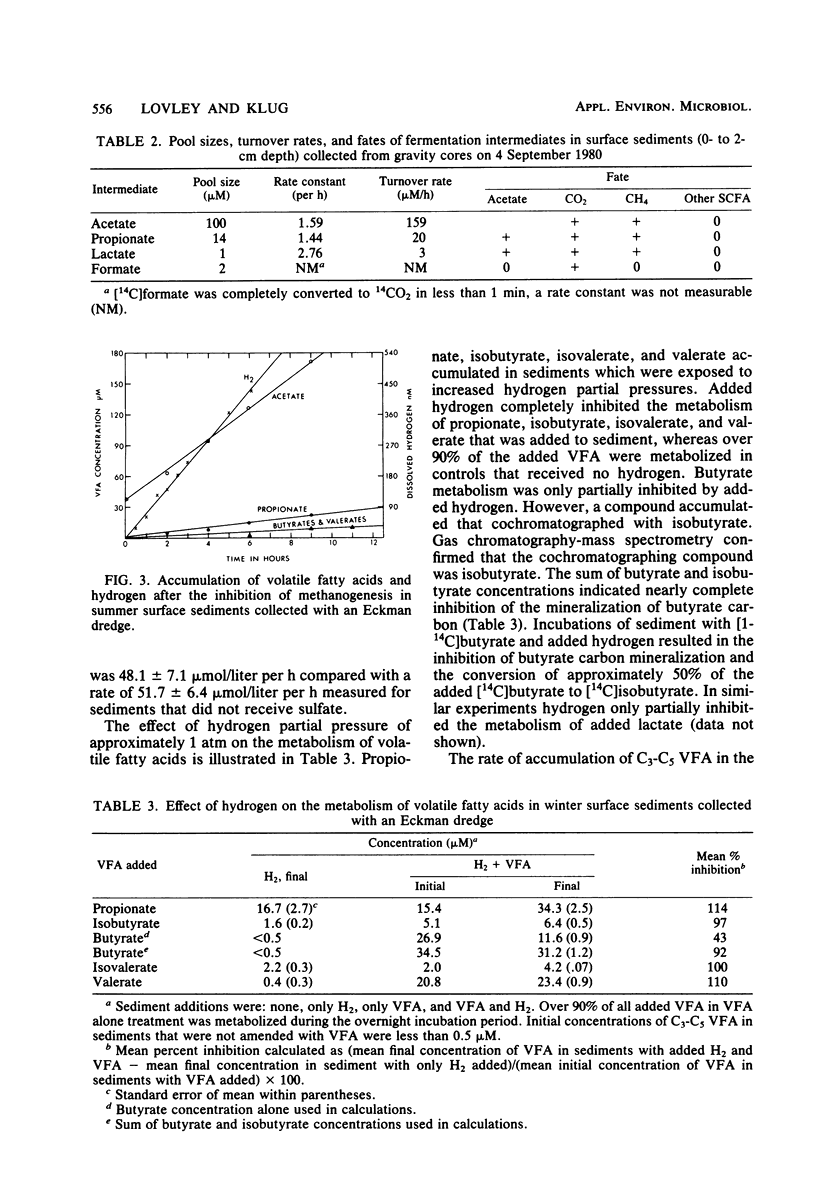
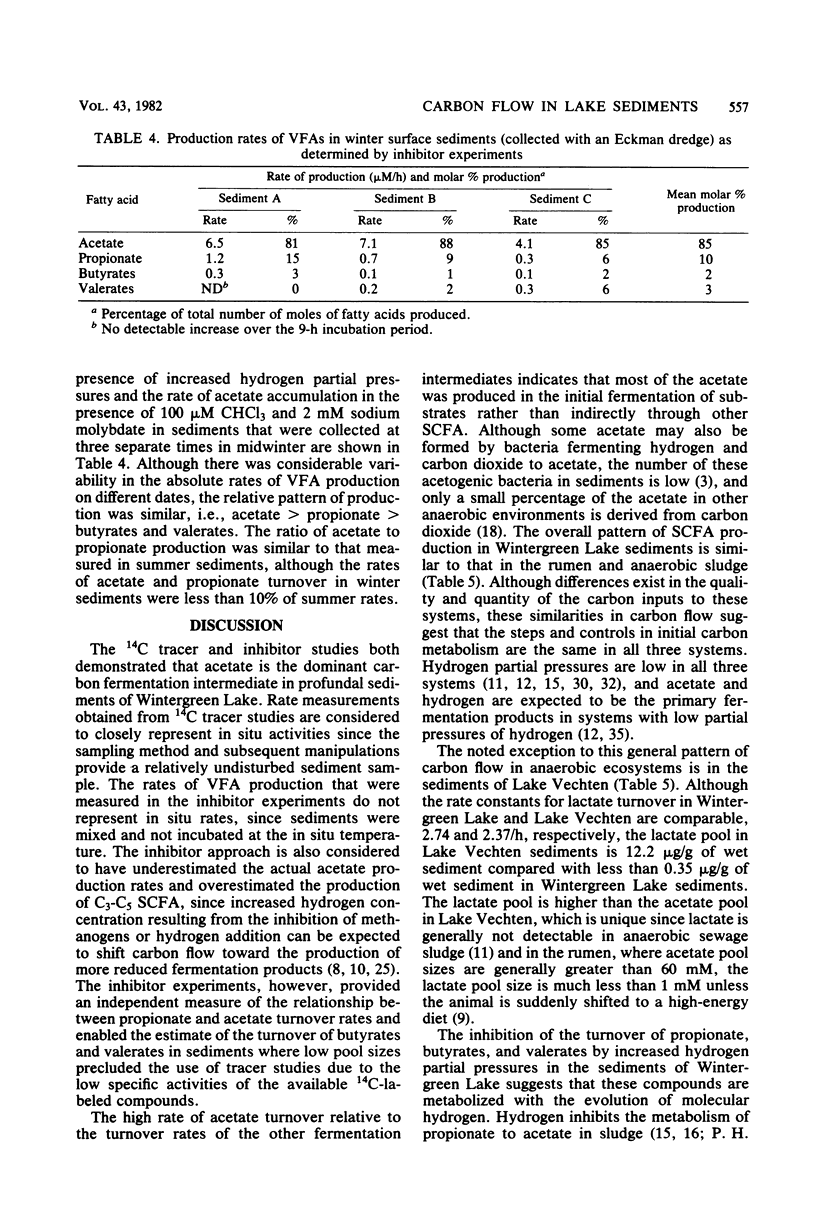
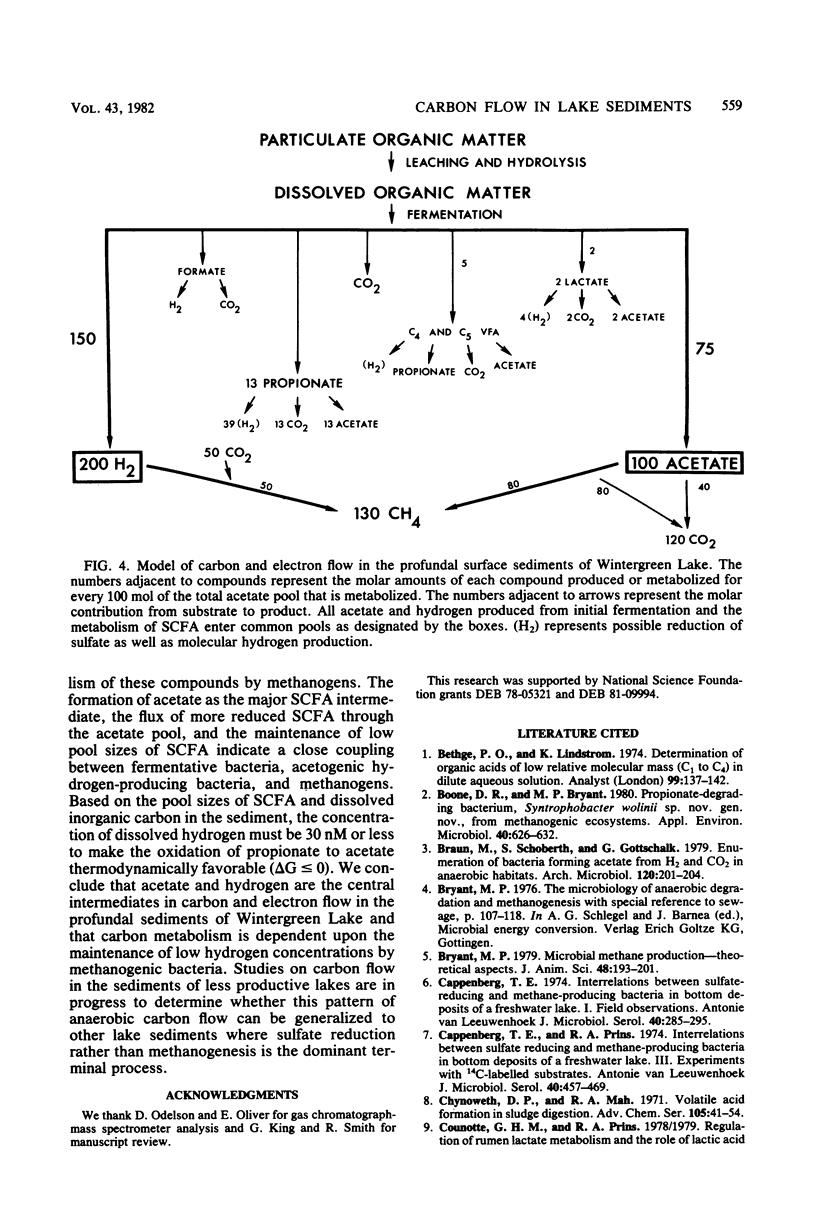
The rates, products, and controls of the metabolism of fermentation intermediates in the sediments of a eutrophic lake were examined. 14C-fatty acids were directly injected into sediment subcores for turnover rate measurements. The highest rates of acetate turnover were in surface sediments (0- to 2-cm depth). Methane was the dominant product of acetate metabolism at all depths. Simultaneous measurements of acetate, propionate, and lactate turnover in surface sediments gave turnover rates of 159, 20, and 3 μM/h, respectively. [2-14C]propionate and [U-14C]lactate were metabolized to [14C]acetate, 14CO2, and 14CH4. [14C]formate was completely converted to 14CO2 in less than 1 min. Inhibition of methanogenesis with chloroform resulted in an immediate accumulation of volatile fatty acids and hydrogen. Hydrogen inhibited the metabolism of C3-C5 volatile fatty acids. The rates of fatty acid production were estimated from the rates of fatty acid accumulation in the presence of chloroform or hydrogen. The mean molar rates of production were acetate, 82%; propionate, 13%; butyrates, 2%; and valerates, 3%. A working model for carbon and electron flow is presented which illustrates that fermentation and methanogenesis are the predominate steps in carbon flow and that there is a close interaction between fermentative bacteria, acetogenic hydrogen-producing bacteria, and methanogens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethge P. O., Lindström K. Determination of organic acids of low relative molecular mass (C1 to C4) in dilute aqueous solution. Analyst. 1974 Feb;99(175):137–142. doi: 10.1039/an9749900137. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Schoberth S., Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979 Mar 12;120(3):201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Reichl J., Prins R. Parameters of rumen fermentation in a continuously fed sheep: evidence of a microbial rumination pool. Appl Microbiol. 1971 Dec;22(6):1104–1113. doi: 10.1128/am.22.6.1104-1113.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAYASURIYA G. C., HUNGATE R. E. Lactate conversions in the bovine rumen. Arch Biochem Biophys. 1959 Jun;82(2):274–287. doi: 10.1016/0003-9861(59)90123-7. [DOI] [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufener W. H., Jr, Wolin M. J. Effect of CCl4 on CH4 and volatile acid production in continuous cultures of rumen organisms and in a sheep rumen. Appl Microbiol. 1968 Dec;16(12):1955–1956. doi: 10.1128/am.16.12.1955-1956.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Christensen D., Jørgensen B. B. Volatile Fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl Environ Microbiol. 1981 Jul;42(1):5–11. doi: 10.1128/aem.42.1.5-11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Anaerobic metabolism of immediate methane precursors in Lake Mendota. Appl Environ Microbiol. 1979 Feb;37(2):244–253. doi: 10.1128/aem.37.2.244-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B., Brock T. D. Measuring radioactive methane with the liquid scintillation counter. Appl Environ Microbiol. 1979 May;37(5):897–899. doi: 10.1128/aem.37.5.897-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


