Abstract
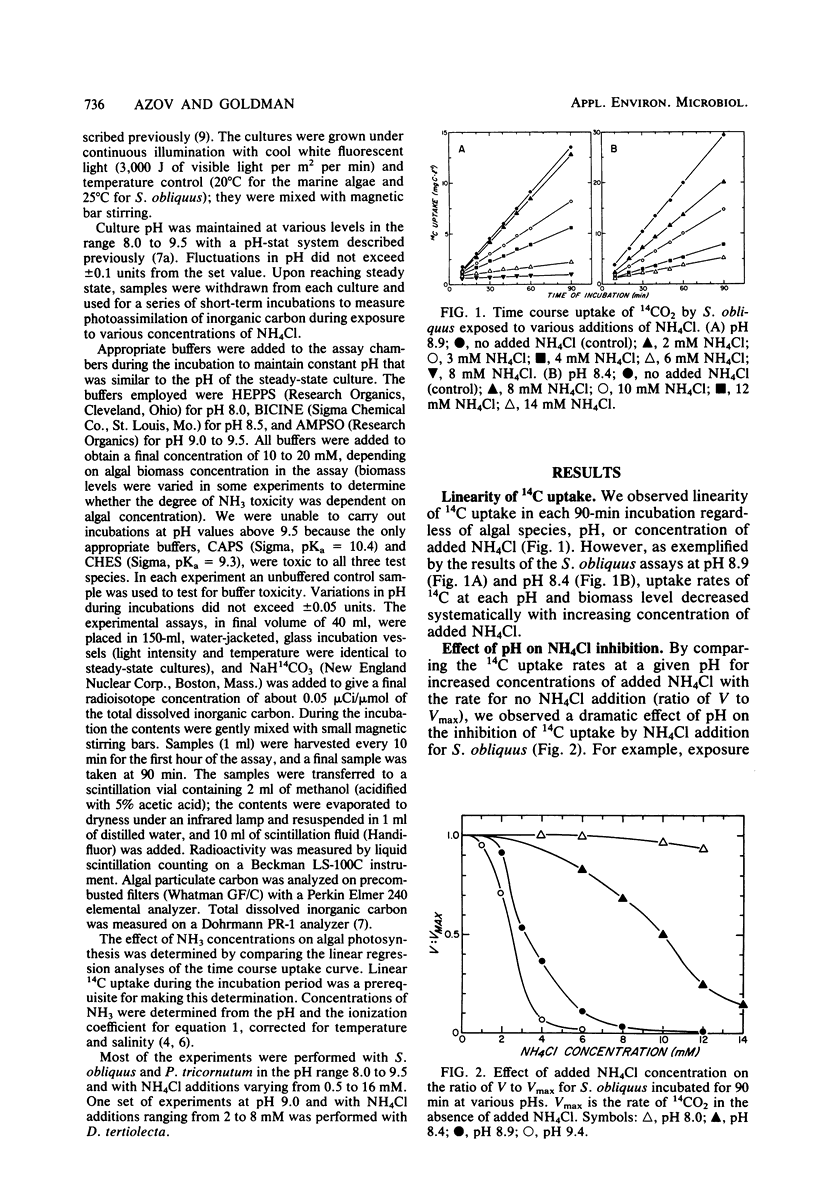
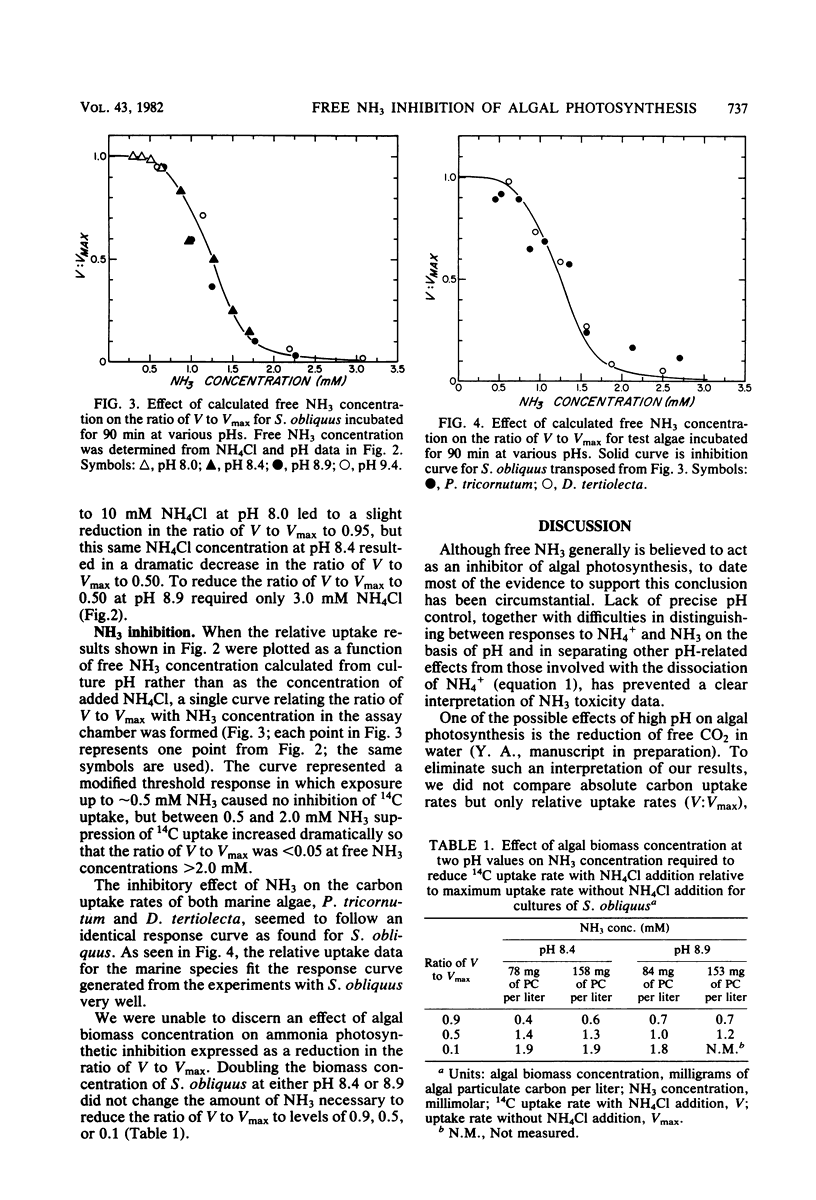
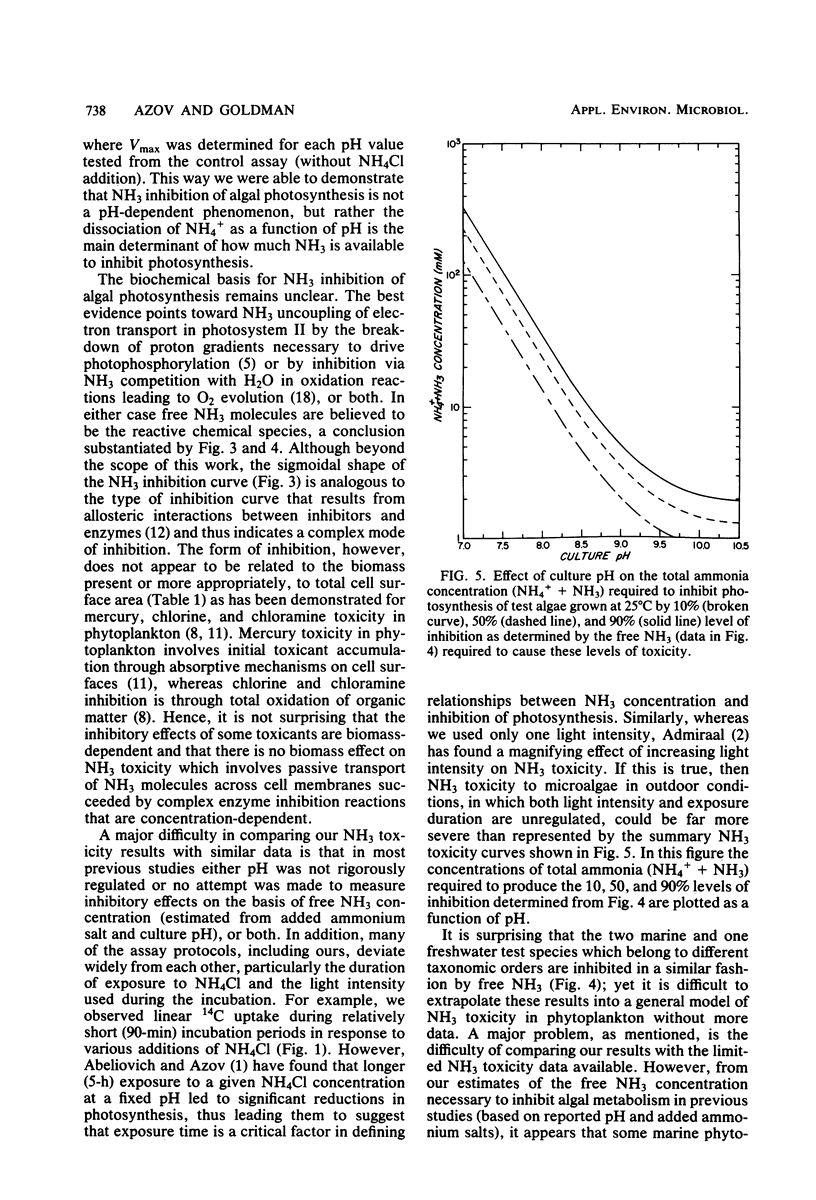
The effect of free NH3 inhibition on short-term photosynthesis was investigated in three microalgal species: the freshwater chlorophyte Scenedesmus obliquus, the marine diatom Phaeodactylum tricornutum and the marine chlorophyte Dunaliella tertiolecta. By performing a series of assays at various concentrations of added NH4Cl and culture pH, we demonstrated that the inhibitory compound was free NH3 and that pH played no role in determining the magnitude of inhibition, other than in establishing the degree of dissociation of nontoxic NH4+ to toxic NH3. When corrections were made for pH, all three species displayed the same sigmoidal response curve to free NH3 concentration; 1.2 mM NH3 led to 50% reduction in photoassimilation of 14C. Based on literature values, some marine phytoplankton appear to be significantly more sensitive to free NH3 than were the test species, which are noted for their excellent growth characteristics. However, the combination of low algal biomass and strong pH buffering commonly found in most marine and many freshwater environments probably limits the possibilities for NH3 toxicity to low alkalinity freshwaters and intensive algal cultures in which NH4+ is the main source of N. Such conditions occur commonly in algal wastewater treatment systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Azov Y. Toxicity of ammonia to algae in sewage oxidation ponds. Appl Environ Microbiol. 1976 Jun;31(6):801–806. doi: 10.1128/aem.31.6.801-806.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- Crofts A. R. Uptake of ammonium ion by chloroplasts, and the mechanism of amine uncoupling. Biochem Biophys Res Commun. 1966 Jul 6;24(1):127–134. doi: 10.1016/0006-291x(66)90420-7. [DOI] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Goldman J. C., Peavey D. G. Steady-State Growth and Chemical Composition of the Marine Chlorophyte Dunaliella tertiolecta in Nitrogen-Limited Continuous Cultures. Appl Environ Microbiol. 1979 Nov;38(5):894–901. doi: 10.1128/aem.38.5.894-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan P. J., Thompson N. P., Patouillet C., Wilkniss P. E. 203Hg as tracer in pigments of Phaeodactylum tricornutum. Int J Appl Radiat Isot. 1973 Dec;24(12):665–670. doi: 10.1016/0020-708x(73)90101-4. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Natarajan K. V. Toxicity of ammonia to marine diatoms. J Water Pollut Control Fed. 1970 May;42(5 Suppl):R184+–R184+. [PubMed] [Google Scholar]
- Raven J. A. Nutrient transport in microalgae. Adv Microb Physiol. 1980;21:47–226. doi: 10.1016/s0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- SHILO MOSHE, SHILO MIRIAM Conditions which determine the efficiency of ammonium sulphate in the control of Prymnesium parvum in fish breeding ponds. Appl Microbiol. 1953 Nov;1(6):330–333. doi: 10.1128/am.1.6.330-333.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


