Abstract
The conserved two-component regulatory system GacS/GacA determines the expression of extracellular products and virulence factors in a variety of Gram-negative bacteria. In the biocontrol strain CHA0 of Pseudomonas fluorescens, the response regulator GacA is essential for the synthesis of extracellular protease (AprA) and secondary metabolites including hydrogen cyanide. GacA was found to exert its control on the hydrogen cyanide biosynthetic genes (hcnABC) and on the aprA gene indirectly via a posttranscriptional mechanism. Expression of a translational hcnA′-′lacZ fusion was GacA-dependent whereas a transcriptional hcnA-lacZ fusion was not. A distinct recognition site overlapping with the ribosome binding site appears to be primordial for GacA-steered regulation. GacA-dependence could be conferred to the Escherichia coli lacZ mRNA by a 3-bp substitution in the ribosome binding site. The gene coding for the global translational repressor RsmA of P. fluorescens was cloned. RsmA overexpression mimicked partial loss of GacA function and involved the same recognition site, suggesting that RsmA is a downstream regulatory element of the GacA control cascade. Mutational inactivation of the chromosomal rsmA gene partially suppressed a gacS defect. Thus, a central, GacA-dependent switch from primary to secondary metabolism may operate at the level of translation.
Keywords: translational control, two-component regulatory system, hydrogen cyanide, biocontrol, virulence
Microorganisms that live in association with plant or animal cells rely extensively on the production of extracellular proteins, secondary metabolites, and siderophores to establish themselves in their habitats. In many Gram-negative bacteria, a conserved two-component regulatory system consisting of the sensor kinase GacS and the cognate response regulator GacA has a decisive role in the control of extracellular products. The sensor GacS (originally designated LemA) was discovered in the plant pathogen Pseudomonas syringae pv. syringae as a factor being necessary for the manifestation of spot lesions on bean leaves (1, 2). The GacA response regulator was first described as a global activator of secondary metabolism and biocontrol activity in the plant-beneficial strain CHA0 of Pseudomonas fluorescens (3, 4). There is ample genetic evidence that the GacS and GacA proteins form a functional pair in a variety of bacterial species (5–7).
In animal pathogens such as Pseudomonas aeruginosa (8, 9), Salmonella typhimurium (10, 11), uropathogenic Escherichia coli (12), and Vibrio cholerae (13), the gacS and gacA homologs are important for virulence. Similarly, in plant pathogens, e.g., P. syringae (1, 2), Pseudomonas tolaasii (14), Erwinia carotovora subsp. carotovora (6, 15), and Xanthomonas campestris pv. campestris (16), mutations in the gacS and gacA homologs result in a nonpathogenic phenotype. Interestingly, a gacA mutant of P. aeruginosa has attenuated virulence in both animals and plants (8, 9). In root-colonizing biocontrol strains of P. fluorescens, the GacS/GacA system determines the expression of extracellular antifungal compounds and the protection of plant roots from fungal pathogens (3, 7, 17, 18).
On interaction with unknown signals, the GacS sensor is presumed to activate the GacA response regulator by phosphorylation. Activated GacA, by virtue of its typical C-terminal helix-turn-helix DNA binding motif (3), is thought to regulate the transcription of target genes. Although the direct GacA targets remain to be identified, it has been shown that the GacS/GacA system exerts a positive effect on cell density-dependent gene regulation mediated by N-acylhomoserine lactones, in at least three bacterial species, P. aeruginosa (19), P. syringae (2), and Pseudomonas aureofaciens (20). However, the GacS/GacA system also operates effectively in some Gram-negative bacteria that are not known to produce N-acylhomoserine lactones (21). For example, in P. fluorescens CHA0, there is no evidence for these signal molecules, and yet the GacS/GacA system strictly controls the expression of extracellular products (antibiotics, exoenzymes, and hydrogen cyanide) when cells are in the transition from exponential to stationary phase (ref. 3; data not shown).
In this study, we show that GacA control of the synthesis of two different exoproducts manifests itself strongly at a posttranscriptional level in P. fluorescens. We present evidence that GacA and RsmA (repressor of secondary metabolism), an RNA binding protein previously studied in enteric bacteria (22, 23), have opposite regulatory effects on the translational expression of target genes. From these results, a model is deduced, according to which the GacS/GacA system can determine virulence or biocontrol activities at the level of translation in Gram-negative bacteria.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
P. fluorescens CHA0 (wild-type), CHA89 (gacA∷Km), and CHA21 (anr∷ΩKm) have been previously described (3, 24). The construction of strains CHA510 (gacS∷Tn5), CHA805 (chromosomal aprA′-′lacZ fusion) and CHA806 (gacS, aprA′-′lacZ) will be reported elsewhere. Strain CHA807 was obtained as described below. Strains CHA207 (chromosomal hcnA′-′lacZ fusion), CHA89.207 (gacA, hcnA′-′lacZ), CHA213 (chromosomal hcn-lacZ fusion), and CHA89.213 (gacA, hcn-lacZ) were constructed by transferring the translational and transcriptional fusions of pME3219 and pME6521, respectively, to the chromosome of strains CHA0 and CHA89, using the suicide vector pME3088 as described (24, 25). Recombinant plasmids were constructed in the vectors pME6000 (26), pME6001 (a gentamicin-resistant pME6000 derivative), pME6010 (GenBank accession no. AF118810), and the cosmid pVK100 (27) and were introduced into P. fluorescens by electroporation (28). P. fluorescens and E. coli strains were grown at 30°C and 37°C, respectively, in nutrient yeast broth or on nutrient agar plates (24). Tetracycline was used at 25 μg/ml (E. coli) or 125 μg/ml (P. fluorescens), and gentamicin at 10 μg/ml.
DNA Manipulation and Cloning Procedures.
DNA cloning and plasmid preparations were performed according to standard methods (29). The promoter probe vector pME6522 carrying the promoterless E. coli lacZ gene (on a 3.3-kilobase PstI-DraI fragment) was derived from pME3533 (30) and the vector pME6010 (Fig. 1). Plasmid pME6521 was obtained by PCR-amplification of the hcn promoter region (260 bp) by using EcoRI-tagged primer 1 (24) and primer 12 (5′-CATGCCTGCAGCAATCTAGTCGGTTTGTCGG-3′), which anneals at the +1 transcription start site and creates an artificial PstI site (underlined). This fragment was fused to lacZ of pME6522 (Fig. 1). Plasmid pME6060–3 (Fig. 1) was constructed by fusing, in frame, the aprA gene on a 3.0-kilobase genomic XhoI-SacI fragment of P. fluorescens CHA0 (S.H., unpublished work) with a ′lacZ fragment (31). Plasmids pME6530 and pME3843 (Fig. 1) were obtained as follows: Plasmid pME3219, which carries a translational hcnA′-′lacZ fusion of strain CHA0 (24), and pME3826, which carries an analogous fusion of P. aeruginosa (G.P., unpublished work), were used as templates to amplify the respective hcnA′ fragments by the use of primers homologous to the first 20 nucleotides of each 5′ leader sequence (5′-GCTCGGTTCTGACAACAGC-3′ and 5′-GCCCGGCCCGACTCCTAGTGT-3′, respectively) and a primer annealing within the lacZ sequence (5′-TGCTGCAAGGCGATTAAGTGGG-3′). The tac promoter of pJF118 (32) was PCR-amplified on a 930-bp fragment by primer 15 (5′-CGAGACAGATCTTAATGGGC-3′), which anneals within the lacI sequence and contains an artificial BglII site (underlined), and primer 16 (5′-GCTCGGTACCCACACATTATACGAGCCGA-3′), which anneals at the +1 transcriptional start and creates a KpnI site (underlined). The tac promoter fragment obtained was inserted into pUK21 (33). This construct was cleaved with KpnI, was treated with T4 DNA polymerase to remove the 3′ overhang, and was linked to either of the blunt-end hcnA′ fragments, which had been cut with PstI at their 3′ ends. The BglII-PstI fragments of the resulting constructs were reintroduced into pME3219 and pME3826, giving pME6530 and pME3843, respectively. This placed the authentic 5′ hcnA′ regions under tac promoter control. The prototype plasmid pME6533 (Fig. 4), which served as a starting point for mutagenesis of the ribosome binding site (RBS), was identical with the translational hcnA′-′lacZ fusion plasmid pME3219, except for the KpnI and SphI restriction sites. These were introduced into pME3219 (Fig. 1) by PCR, using primers 1 (24) and 17 (5′-ATGAATGGGGTACCCGGCGTCCCG-3′), which anneals to the RBS region of P. fluorescens hcnA and contains two nucleotide changes resulting in a KpnI site (underlined). This 340-bp PCR fragment was joined to a 70-mer, which was bordered by a 5′ KpnI and a 3′ PstI site and consisted of the hcnA sequence, except for two nucleotides creating a SphI site (Fig. 4), and was inserted into pME3219 cleaved with EcoRI and PstI. The derivatives of pME6533 were constructed by exchanging 32-mer KpnI-SphI linkers carrying different sequence modifications. The three-base substitution in the plasmid pME6544, which is otherwise identical with pME6521, was generated by the overlap extension method (34), using primer 19 (5′-TTCACGGATGAAACAGCTATGACCA-3′) to introduce the mutation (underlined). The rsmA gene of P. fluorescens was cloned as explained in Fig. 5. This gene was inactivated by insertion of the Ω-Km element (35) into the unique FspI site and marker exchanged (24) with the chromosomal rsmA gene of strain CHA806 (gacS, aprA′-′lacZ), resulting in strain CHA807.
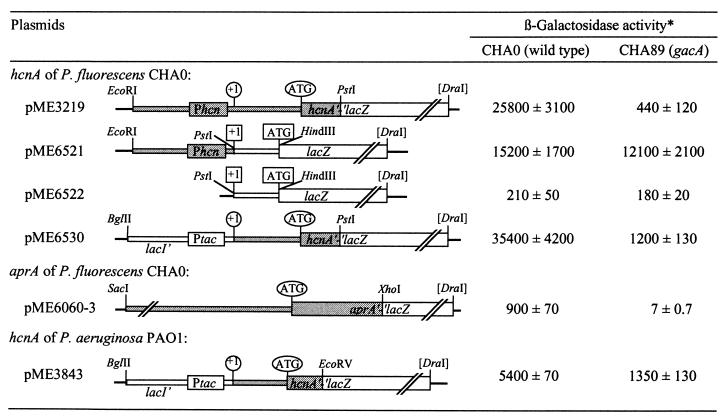
Figure 1.
Posttranscriptional, gacA-dependent regulation of the Pseudomonas hcnA and aprA genes in P. fluorescens. All constructs were made in the vector pME6010. *, β-galactosidase expression (Miller units) of the lacZ fusions shown was tested in the P. fluorescens strains CHA0 and CHA89 when cells reached an OD600 of 2.0–2.5. Activities are mean values of triplicate experiments ± standard deviation.
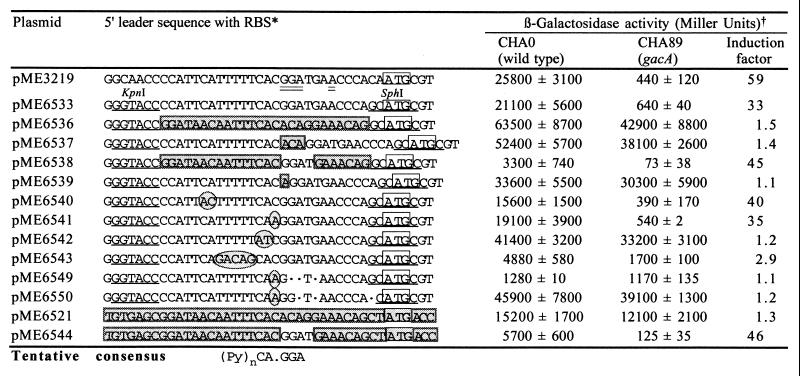
Figure 4.
Posttranscriptional, GacA-dependent regulation of a translational hcnA′-′lacZ fusion (derived from the P. fluorescens hcn operon): influence of mutations in the 5′ leader sequence. *, Nucleotides of importance are designated as follows: doubly underlined, RBS; underlined, artificial restriction sites; shaded boxes, E. coli lacZ sequence; open box, ATG initiation codon of hcnA; encircled, base substitutions; ●, deletion. †, β-galactosidase expression (Miller units) was determined in strains CHA0 and CHA89, when cells reached an OD600 of 2.0–2.5. Activities are mean values from triplicate experiments ± standard deviation.
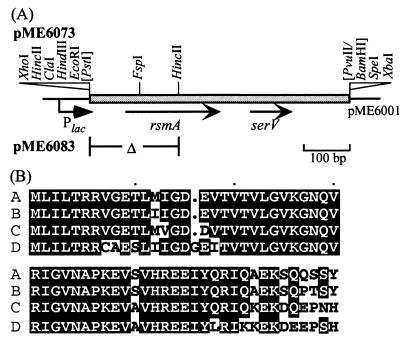
Figure 5.
(A) Cloning of the rsmA gene of P. fluorescens CHA0. The P. aeruginosa rsmA gene was isolated by PCR and was used as a probe to identify the homolog in P. fluorescens by Southern hybridization. A 1.25-kilobase genomic PstI fragment containing rsmA of P. fluorescens was cloned. In pME6073, rsmA on a 0.55-kilobase PstI/PvuII fragment is expressed from the lac promoter of the vector pME6001; in pME6083, rsmA is deleted. The serV gene, which encodes a serine tRNA, appears to belong to a separate transcription unit. (B) Alignment of RsmA and CsrA amino acid sequences. A, CsrA of E. coli (GenBank accession no. L07596); B, RsmA of E. carotovora subsp. carotovora 71 (L40173); C, RsmA of P. aeruginosa (AF061757); D, RsmA of P. fluorescens. Amino acid residues that are conserved in at least three of the four proteins are shown in black boxes.
Southern Hybridization and Nucleotide Sequence Analyses.
Southern blotting of P. fluorescens genomic DNA was performed as described (19). The nucleotide sequence of the rsmA gene of P. fluorescens (GenBank accession no. AF136151) as well as the products of all PCR and linker constructs were determined and verified by using the Dye Terminator Kit (Perkin–Elmer, 402080) and the Applied Biosystems PRISM 373 sequencer. Nucleotide and deduced amino acid sequences were analyzed with the programs blast, gap, bestfit, and pileup by using the Genetics Computer Group (Madison, WI) package.
β-Galactosidase Assay.
For β-galactosidase measurements, P. fluorescens cells were grown with shaking in 50-ml flasks containing 20 ml of nutrient yeast broth at 30°C. β-Galactosidase specific activities were determined by the Miller method (29).
Results
Regulation of the hcnABC Operon in P. fluorescens CHA0.
The expression of the hydrogen cyanide biosynthetic genes hcnABC requires the transcription factors GacA and ANR in P. fluorescens (3, 24). The anaerobic regulator ANR activates transcription of the hcnABC genes by binding to the −40 region of the hcnA promoter (24). The sites of GacA control have not been determined previously. A translational hcnA′-′lacZ fusion on plasmid pME3219 (Fig. 1) was used to quantify the regulatory effects of GacS/GacA and ANR in P. fluorescens CHA0. In both a gacS and a gacA mutant, hcnA′-′lacZ expression was reduced ≈50-fold, compared with the level in the wild-type CHA0. In an anr mutant, hcnA′-′lacZ expression was very low, at the detection limit (Table 1). To see whether GacS/GacA and ANR might be elements of a common regulatory cascade, we tested translational gacA′-′lacZ and anr′-′lacZ fusions in the wild type, in an anr mutant, and in a gacA mutant. The expression of both fusions was similar in the three strains (data not shown), suggesting that ANR and GacA are not in a common cascade and show little, if any, autoregulation. We (C.B. and D.H., unpublished work) have obtained evidence that transcriptional regulation of hcnABC by oxygen and iron availability depends on ANR and the −40 promoter region and that GacA (or a regulator controlled by GacA) activates cyanogenesis independently of ANR.
Table 1.
Regulation of a translational hcnA′-′lacZ fusion on pME3219 by the gacS/gacA and anr genes in P. fluorescens
| Host* | Relevant genotype | β-galactosidase activity, (Miller units)† |
|---|---|---|
| CHA0 | wild type | 25,800 ± 3,100 |
| CHA89 | gacA∷Km | 440 ± 120 |
| CHA510 | gacS∷Tn5 | 490 ± 100 |
| CHA21 | anr∷Ω-Km | 5 ± 0.5 |
Each host strain carried pME3219. β-galactosidase activity (Miller units) was determined when cells were mildly oxygen-limited at an OD600 of 2.0–2.5. Under these conditions, the hcnA promoter is partially induced.
†Means of three or more independent experiments (± standard deviation).
GacS/GacA Control of the hcn Biosynthetic Genes Manifests Itself at a Posttranscriptional Level.
The promoter probe vector pME6522 was used to construct a transcriptional hcn-lacZ fusion, in which the hcn promoter was linked, at the transcription start site, to the E. coli lacZ gene with its own 5′ leader sequence and ribosome binding site (RBS) (Fig. 1). Expression of the transcriptional hcn-lacZ fusion (on pME6521) was similar in the wild-type CHA0 and in the gacA mutant CHA89 whereas the translational hcnA′-′lacZ fusion (on pME3219) strongly depended on GacA function (Fig. 1). β-galactosidase expression of the lacZ reporter pME6522 was negligibly low and not influenced by GacA (Fig. 1). To rule out copy-number artifacts (the vector pME6010 has about six copies in P. fluorescens), we transferred the translational and transcriptional lacZ fusions of pME3219 and pME6521, respectively, to the chromosome of strains CHA0 and CHA89 (gacA). The same pattern of GacA-dependent hcn regulation was observed (Table 2). Furthermore, the plasmid-borne translational hcnA′-′lacZ fusion remained essentially under GacA control when the anaerobically inducible hcn promoter was replaced by the constitutively expressed tac promoter (on pME6530; Fig. 1). In this construct, the 5′ untranslated hcnA leader sequence was the same as in the native hcnABC operon. Irrespective of the promoter used, the hcnA′-′lacZ translational fusions on both pME3219 and pME6530 showed similar growth phase-dependent expression, with maximal levels reached at the end of exponential growth phase (Fig. 2). Taken together, these results suggest that the GacS/GacA two-component system regulates hcn expression at a posttranscriptional level, in response to the growth phase and/or cell density.
Table 2.
Expression of the chromosomal hcn genes of P. fluorescens
| Strain | Genotype | β-galactosidase activity, Miller units* |
|---|---|---|
| CHA207 | Translational hcnA′-′lacZ fusion | 1,515 ± 140 |
| CHA89.207 | Translational hcnA′-′lacZ fusion, gacA∷Km | 35 ± 2 |
| CHA213 | Transcriptional hcn-lacZ fusion | 2,065 ± 89 |
| CHA89.213 | Transcriptional hcn-lacZ fusion, gacA∷Km | 1,825 ± 52 |
Conditions were the same as in Table 1; measurements were taken at an OD600 of 2.
Figure 2.
Cell density-dependent expression of a translational hcnA′-′lacZ fusion from the hcn promoter on pME3219 (circles) or from the tac promoter on pME6530 (squares) in CHA0 (open symbols) and CHA89 (closed symbols). Bacterial growth reached a plateau at OD600 4–5.
Evidence for a General GacS/GacA Control Mechanism.
In P. fluorescens CHA0, the expression of the major extracellular protease is under strict GacA control (4). The structural gene, aprA, for this metalloprotease was cloned (S.H., unpublished data). A translational aprA′-′lacZ fusion (on pME6060-3) was tightly regulated by GacA in P. fluorescens, with an induction factor of >100 (Fig. 1). We also included in this analysis the P. aeruginosa hcnABC cluster, which is 77% identical to the hcnABC operon of P. fluorescens (G.P., unpublished results). The hcnA promoter of P. aeruginosa is expressed under the control of N-butyryl-homoserine lactone, whose level is regulated by GacA in the native host (19). In strain CHA0, the hcnA promoter of P. aeruginosa was poorly expressed (data not shown). Therefore, we replaced this promoter by the tac promoter. Unexpectedly, the P. aeruginosa hcnA′-′lacZ fusion (expressed from ptac, on pME3843) was still regulated, albeit weakly, by GacA in P. fluorescens CHA0 (Fig. 1). A sequence alignment of the 5′ untranslated leader regions of the hcnA genes of P. fluorescens and P. aeruginosa and of the aprA gene of P. fluorescens revealed some similarity in the vicinity of the RBS (Fig. 3). We therefore postulated that this region could be a common target for GacA control. The E. coli lacZ gene, whose expression was not modulated by GacA in P. fluorescens (Fig. 1), was included in the alignment (Fig. 3), to narrow down the most important zone of similarity among the three Pseudomonas sequences.
Figure 3.
Alignment of the regions containing the RBS of the hcnA genes of P. fluorescens CHA0 and P. aeruginosa PAO1, the aprA gene of P. fluorescens CHA0, and the E. coli lacZ gene. In each case, translation is initiated at the ATG codon shown at the 3′ end.
Mutational Analysis of the RBS Region of the P. fluorescens hcnA Gene.
Guided by the sequence comparison (Fig. 3), we introduced specific mutations into a 33-bp segment carrying the RBS of the P. fluorescens hcnA gene, using the translational hcnA′-′lacZ fusion on pME3219 (Fig. 1) as a reporter. To facilitate mutagenesis, we first created artificial restriction sites for KpnI and SphI bracketing the target region. The resulting construct, pME6533, remained under GacA control (Fig. 4). However, when the hcn target region was substituted by the equivalent lac segment (on pME6536), no GacA-dependent regulation was observed (Fig. 4). (The induction factor of 1.5 measured for pME6536 was considered to be insignificant.) Insertion of three strategically placed nucleotides (ACA), which are characteristic of the lacZ leader, sufficed to abolish GacA control of the hcnA′-′lacZ fusion in pME6537 (Fig. 4). The ACA insertion altered the spacing between the presumed RBS (GGA) and several conserved pyrimidines located upstream (Fig. 4). Next, a critical substitution was introduced into the lacZ leader carried by pME6538: the lac-specific nucleotides ACAG were exchanged for the hcn-specific tetrad GGAT. This substitution installed GacA control (Fig. 4), presumably because the correct spacing between the conserved pyrimidines and the RBS (GGA) was restored.
Several further constructs were made to confirm these observations. Addition of a single base (A) to the hcn sequence changed the critical spacing (on pME6539) and abolished GacA control (Fig. 4). In the construct pME6540, two bases were exchanged (CA→AC) to disrupt a potential secondary mRNA structure. However, this change had no influence on GacA control (Fig. 4). Another substitution (C→A), which created an RBS typical of aprA (Fig. 3), did not affect GacA control either (on pME6541; Fig. 4). By contrast, a substitution (CA→AT) that modified the central conserved CA.GGA motif (Fig. 3) abolished GacA control (on pME6542; Fig. 4). Two further changes of the central motif, i.e., the deletions in pME6549 and pME6550, similarly interfered with GacA control. The conserved pyrimidines upstream of the central motif appear to make some contribution to GacA control, as evidenced by pME6543 (Fig. 4). Although the translational ′lacZ fusions of the constructs shown in Fig. 4 were expressed at widely different levels because of variable translation efficiencies, induction factors of >30 or <2 clearly indicated the presence or absence of GacA control, respectively.
Is the segment lying between the KpnI and SphI sites sufficient to promote GacA control? To answer this question, the GacA-independent hcn-lacZ transcriptional fusion on pME6521 (Fig. 1) was mutated at the RBS by exchanging the lac-specific nucleotides ACAG by the hcn counterpart GGAT (on pME6544). This substitution of three nucleotides in the lacZ 5′ leader sequence was enough to establish regulation by GacA (Fig. 4). A preliminary and tentative consensus sequence based on the alignments of Figs. 3 and 4 is postulated for GacA control (Fig. 4).
Cloning of the Global Translational Repressor RsmA of P. fluorescens.
In E. carotovora, RsmA is a global negative regulator of genes encoding extracellular products and virulence factors (36). The small RNA-binding protein RsmA, like its homolog CsrA of E. coli, is assumed to recognize a region at or close to the RBS of susceptible mRNA molecules (22, 23). Strains overexpressing rsmA or being devoid of expA (=gacA) have similar pleiotropic phenotypes in Erwinia spp. (6, 36). To see whether the same holds true for P. fluorescens, we cloned and sequenced the rsmA gene of strain CHA0 (Fig. 5). The RsmA proteins of P. fluorescens, P. aeruginosa, and E. carotovora and CsrA of E. coli were found to be highly conserved (Fig. 5), suggesting a common mode of action.
GacA and Overexpressed RsmA Act on the Same Specific RBS.
The rsmA gene of P. fluorescens was placed under the control of the lac promoter in the multicopy plasmid pME6073 (Fig. 5). This plasmid repressed the expression of chromosomal hcnA′-′lacZ and aprA′-′lacZ fusions in P. fluorescens 7-fold and 20-fold, respectively. A pME6073 derivative (pME6083) in which most of the rsmA gene had been deleted (Fig. 5) did not repress the hcnA′-′lacZ and aprA′-′lacZ fusions (data not shown). To test whether the overexpressed rsmA gene could mimick a gacA defect, we chose 10 representative hcnA′-′lacZ fusion plasmids containing different RBS modifications (Fig. 4) and assayed β-galactosidase activities with or without extra rsmA copies (pME6073 or vector pME6001, respectively; Table 3). Each construct that was controlled by GacA was repressible at least 3-fold by overexpressed RsmA; conversely, each GacA-independent construct had an RsmA repression factor of <1.5 (Table 3). This provides strong evidence that regulation by GacA and RsmA acts on essentially the same specific RBS and that both regulators are elements of the same regulatory cascade.
Table 3.
Strict correlation between GacA- and RsmA-dependent regulation in P. fluorescens
| Strain/plasmid* | β-galactosidase
activity†
|
RsmA repression factor | GacA induction factor‡ | |
|---|---|---|---|---|
| + pME6001, vector control | + pME6073, rsmA++ | |||
| CHA0/pME3219 | 12,400 ± 1,000 | 2,600 ± 500 | 4.8 | 59 |
| CHA0/pME6530 | 27,200 ± 1,500 | 6,400 ± 450 | 4.0 | 30 |
| CHA0/pME6533 | 14,400 ± 700 | 2,600 ± 80 | 5.5 | 33 |
| CHA0/pME6536 | 46,800 ± 1,500 | 36,000 ± 2,300 | 1.3 | 1.5 |
| CHA0/pME6537 | 39,300 ± 1,900 | 29,700 ± 3,000 | 1.3 | 1.4 |
| CHA0/pME6538 | 4,300 ± 200 | 830 ± 40 | 5.2 | 45 |
| CHA0/pME6539 | 29,400 ± 900 | 20,000 ± 1,200 | 1.5 | 1.1 |
| CHA0/pME6541 | 12,400 ± 500 | 2,100 ± 140 | 6.0 | 35 |
| CHA0/pME6521 | 7,800 ± 510 | 7,600 ± 290 | 1.0 | 1.3 |
| CHA0/pME6544 | 3,400 ± 70 | 1,000 ± 30 | 3.4 | 46 |
The hcnA′-′lacZ fusion constructs (Figs. 1 and 4) were tested in the presence (pME6073) or absence (pME6001) of overexpressed RsmA. Cells were grown in 20 ml of nutrient yeast broth with gentamicin (10 μg/ml) to an OD600 of 2.0–2.5.
† β-galactosidase activities (Miller units) were determined in triplicate; mean values ± standard deviation are given.
‡Values are from Fig. 4.
Given that RsmA is a negative control element, it can be predicted that an rsmA mutation should suppress a defect in the gacS or gacA gene. Therefore, the chromosomal rsmA gene of the gacS mutant CHA806, which contains a chromosomal aprA′-′lacZ fusion, was inactivated by the insertion of a resistance cassette. The resulting mutant CHA807 grown in nutrient yeast broth to an OD600 of 2–2.5 expressed β-galactosidase activity (180 ± 20 Miller units) whereas no activity (<5 Miller units) was detected in the parental strain CHA806. In strain CHA805 (gacS+) the chromosomal aprA′-′lacZ fusion gave a 3-fold higher β-galactosidase expression (550 ± 90 Miller units). Thus, an rsmA mutation can compensate, at least in part, for the effect of a gacS mutation on aprA expression in P. fluorescens, supporting our model according to which the GacS/GacA system can antagonize the repression by RsmA (see Discussion). However, additional unidentified regulatory elements under GacS/GacA control might be involved in the regulation of the aprA and hcn genes.
Discussion
The most important result of this study is that the widely conserved GacS/GacA two-component system determines the production of an extracellular protein and a secondary metabolite (hydrogen cyanide) at a posttranscriptional level and that small mutational changes in the RBS of target transcripts are sufficient to install or abolish GacA-mediated control. For instance, substitution of three nucleotides in the E. coli lacZ leader mRNA near the RBS sufficed to place the expression of the lacZ gene under GacA control in P. fluorescens (Fig. 4). After this change, lacZ behaved like the aprA and hcn genes. Conversely, substitution or insertion of single strategically located nucleotides in the hcn leader mRNA of P. fluorescens could completely eliminate GacA control (Fig. 4). Thus, a few nucleotides can operate a critical switch from primary to secondary metabolism, and it is likely that this situation applies not only to P. fluorescens but to a wide range of Gram-negative bacteria. In pathogenic species, the same switch may be vital for the regulation of virulence factors.
Because the region responsible for GacA control overlaps with the RBS of hcnA and aprA, this region could comprise a binding site for a regulatory protein: e.g., a translational repressor such as RsmA of E. carotovora and CsrA of E. coli (22, 23). By cloning and overexpressing the rsmA gene of P. fluorescens, we have obtained evidence that regulation by GacA and RsmA depend on essentially the same specific RBS regions, which could constitute RsmA binding sites. This leads to a model that, in its simplest form, predicts that GacA could up-regulate a regulatory macromolecule alleviating RsmA-mediated translational repression. It is possible that relief of repression could be exerted by an RsmB-like RNA molecule. It is known that the action of the translational repressors RsmA in E. carotovora and CsrA in E. coli is antagonized by the regulatory, noncoding RNAs RsmB and CsrB, respectively. These RNAs bind and sequester the repressor proteins (22, 23). An rsmB-negative mutant of E. carotovora has a pleiotropic phenotype resembling that of an expA (gacA)-negative strain (6, 22). In P. fluorescens, binding of RsmA to target mRNAs remains to be demonstrated. Whereas the RsmA/CsrA proteins are very similar in different bacteria (Fig. 5; ref. 23), the RsmB/CsrB RNAs appear to be much less conserved. For instance, our homology searches have failed to reveal an rsmB gene in P. aeruginosa.
Several extensions of our basic model can be envisaged. First, it is possible that RsmA recognition sites could also be located outside the RBS, in coding or noncoding sequences of target genes. Second, in enteric bacteria, RsmA and CsrA can enhance the degradation of target mRNAs (23), and RsmA of P. fluorescens might have the same effect. Third, the GacS/GacA system might directly affect rsmA expression.
Regulatory elements intervening between GacA and target genes can complicate the regulatory cascade. For instance, in P. aeruginosa, the rhlI gene encoding N-butyryl-homoserine lactone synthase appears to be posttranscriptionally regulated by GacA and RsmA (G.P., unpublished data). In turn, N-butyryl-homoserine lactone activates the transcription of hcnABC and other target genes, presumably by binding to the transcriptional regulator RhlR (19, 37). As an overall consequence, hcn expression would seem to be GacA-regulated at the transcriptional level. Such complications might explain some results obtained in P. aureofaciens (20) and E. carotovora (6) in which GacA appears to influence the transcription of target genes. However, our present results do not rule out direct transcriptional control of certain target genes by GacA. In P. fluorescens CHA0, this possibility has been excluded in the case of the hcnA promoter whose expression is totally independent of GacA (C.B. and D.H., unpublished work).
As a rule in Gram-negative bacteria, typical genes of primary metabolism are expressed during different growth phases but especially in the course of exponential growth. By contrast, typical genes of secondary metabolism are predominantly expressed during the idiophase: i.e., the transition from the exponential to stationary phase (Fig. 2; ref. 6). By manipulating the RBS sequences of selected genes, as demonstrated in Fig. 4, it has become possible to target translational gene expression specifically to the idiophase.
Acknowledgments
We thank Christine von Schroetter for help with some preliminary experiments and Cornelia Reimmann and Geneviève Défago for discussion. This work was supported by the Swiss National Science Foundation (projects 31-45896.95 and 31-50522.97), European Union projects BIO4CT960119 and BIO4CT960027, and the Swiss Priority Program Biotechnology (project 5002-04502311).
Abbreviation
- RBS
ribosome binding site(s)
Footnotes
References
- 1.Hrabak E M, Willis D K. J Bacteriol. 1992;174:3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitten T, Kinscherf T G, McEvoy J L, Willis D K. Mol Microbiol. 1998;28:917–929. doi: 10.1046/j.1365-2958.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- 3.Laville J, Voisard C, Keel C, Maurhofer M, Défago G, Haas D. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacherer P, Défago G, Haas D. FEMS Microbiol Lett. 1994;116:155–160. doi: 10.1111/j.1574-6968.1994.tb06694.x. [DOI] [PubMed] [Google Scholar]
- 5.Rich J J, Kinscherf T G, Kitten T, Willis D K. J Bacteriol. 1994;176:7468–7475. doi: 10.1128/jb.176.24.7468-7475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson A R, Andersson R A, Pirhonen M, Palva E T. Mol Plant–Microbe Interact. 1998;11:743–752. doi: 10.1094/MPMI.1998.11.8.743. [DOI] [PubMed] [Google Scholar]
- 7.Whistler C A, Corbell N A, Sarniguet A, Ream W, Loper J E. J Bacteriol. 1998;180:6635–6641. doi: 10.1128/jb.180.24.6635-6641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahme L G, Tan M W, Le L, Wong S M, Tompkins R G, Calderwood S B, Ausubel F M. Proc Natl Acad Sci USA. 1997;94:13245–13250. doi: 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan M W, Mahajan-Miklos S, Ausubel F M. Proc Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston C, Pegues D A, Hueck C J, Lee A, Miller S I. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahmer B M M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J P, Normark S. Science. 1996;273:1234–1246. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]
- 13.Wong S M, Carroll P A, Rahme L G, Ausubel F M, Calderwood S B. Infect Immun. 1998;66:5854–5861. doi: 10.1128/iai.66.12.5854-5861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal S I, Han B, Johnstone K. J Bacteriol. 1995;177:4658–4668. doi: 10.1128/jb.177.16.4658-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederick R D, Chiu J, Bennetzen J L, Handa A K. Mol Plant–Microbe Interact. 1997;10:407–415. doi: 10.1094/MPMI.1997.10.3.407. [DOI] [PubMed] [Google Scholar]
- 16.Barber C E, Tang J L, Feng J X, Pan M Q, Wilson T J, Slater H, Dow J M, Williams P, Daniels M J. Mol Microbiol. 1997;24:555–566. doi: 10.1046/j.1365-2958.1997.3721736.x. [DOI] [PubMed] [Google Scholar]
- 17.Gaffney T D, Lam S T, Ligon J, Gates K, Frazelle A, Di Maio J, Hill S, Goodwin S, Torkewitz N, Allshouse A M, et al. Mol Plant–Microbe Interact. 1994;7:455–463. doi: 10.1094/mpmi-7-0455. [DOI] [PubMed] [Google Scholar]
- 18.Corbell N, Loper J E. J Bacteriol. 1995;177:6230–6236. doi: 10.1128/jb.177.21.6230-6236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 20.Chancey S T, Wood D W, Pierson L S. Appl Environ Microbiol. 1999;65:2294–2299. doi: 10.1128/aem.65.6.2294-2299.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuqua C, Greenberg E P. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Cui Y, Mukherjee A, Chatterjee A K. Mol Microbiol. 1998;29:219–234. doi: 10.1046/j.1365-2958.1998.00924.x. [DOI] [PubMed] [Google Scholar]
- 23.Romeo T. Mol Microbiol. 1998;29:1321–1330. doi: 10.1046/j.1365-2958.1998.01021.x. [DOI] [PubMed] [Google Scholar]
- 24.Laville J, Blumer C, Von Schroetter C, Gaia V, Défago G, Keel C, Haas D. J Bacteriol. 1998;180:3187–3196. doi: 10.1128/jb.180.12.3187-3196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voisard C, Bull C T, Keel C, Laville J, Maurhofer M, Schnider U, Défago G, Haas D. In: Molecular Ecology of Rhizosphere Microorganisms. O’Gara F, Dowling D N, Boesten B, editors. Weinheim, Germany: VCH; 1994. pp. 67–89. [Google Scholar]
- 26.Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D, Défago G. Phytopathology. 1998;88:678–684. doi: 10.1094/PHYTO.1998.88.7.678. [DOI] [PubMed] [Google Scholar]
- 27.Knauf V C, Nester E W. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 28.Farinha M A, Kropinski A M. FEMS Microbiol Lett. 1990;58:221–225. doi: 10.1111/j.1574-6968.1990.tb13982.x. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 30.Winteler H, Haas D. Microbiology. 1996;142:685–693. doi: 10.1099/13500872-142-3-685. [DOI] [PubMed] [Google Scholar]
- 31.Minton N P. Gene. 1984;31:269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 32.Fürste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 33.Vieira J, Messing J. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 34.Mikaelian I, Sergeant A. In: Methods in Molecular Biology. Trower M K, editor. Vol. 57. Totowa, NJ: Humana Press; 1996. pp. 193–202. [DOI] [PubMed] [Google Scholar]
- 35.Fellay R, Frey J, Krisch H. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee A, Cui Y, Liu Y, Dumenyo C K, Chatterjee A K. Appl Environ Microbiol. 1995;61:1959–1967. doi: 10.1128/aem.61.5.1959-1967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S, Lazdunski A, Williams P. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]