Abstract
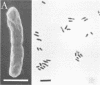
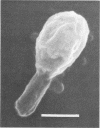
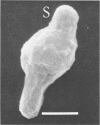
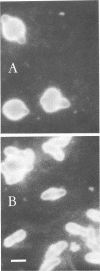
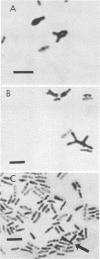
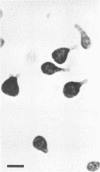
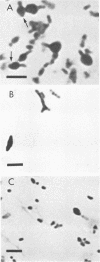
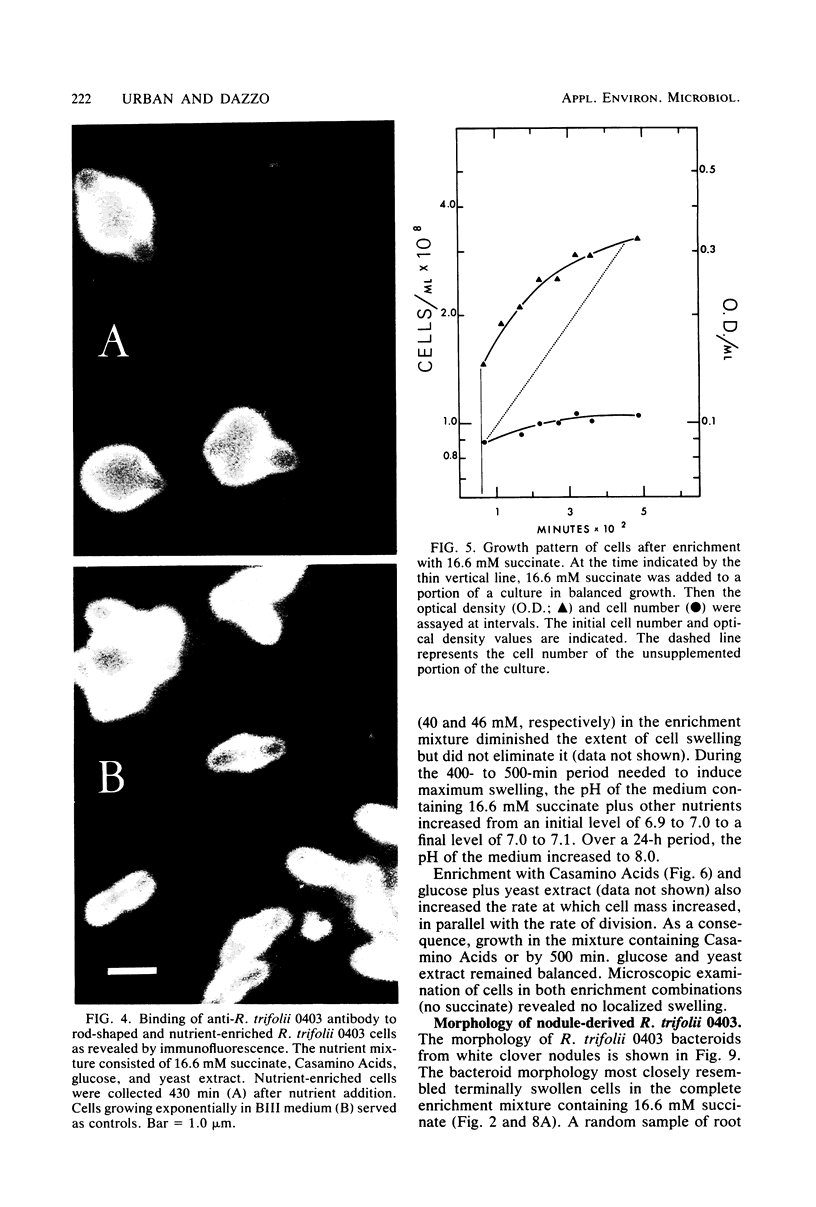
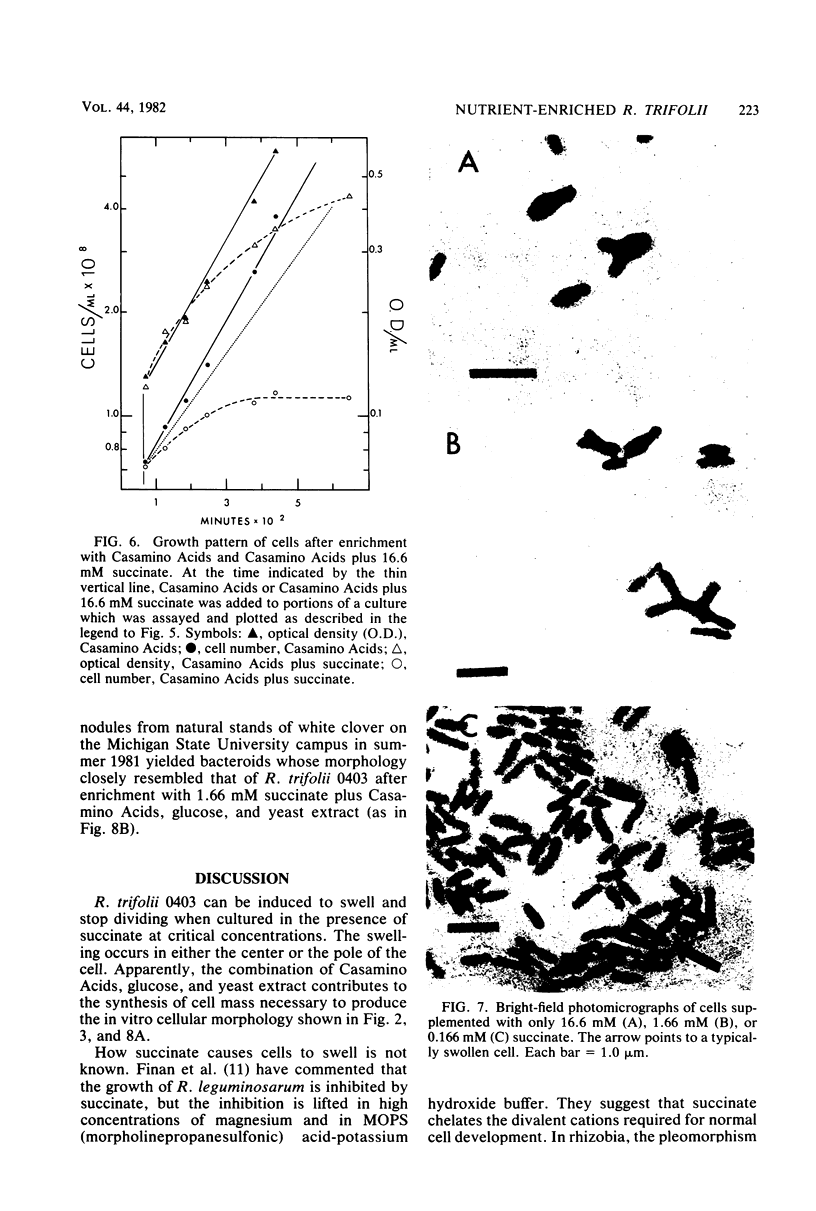
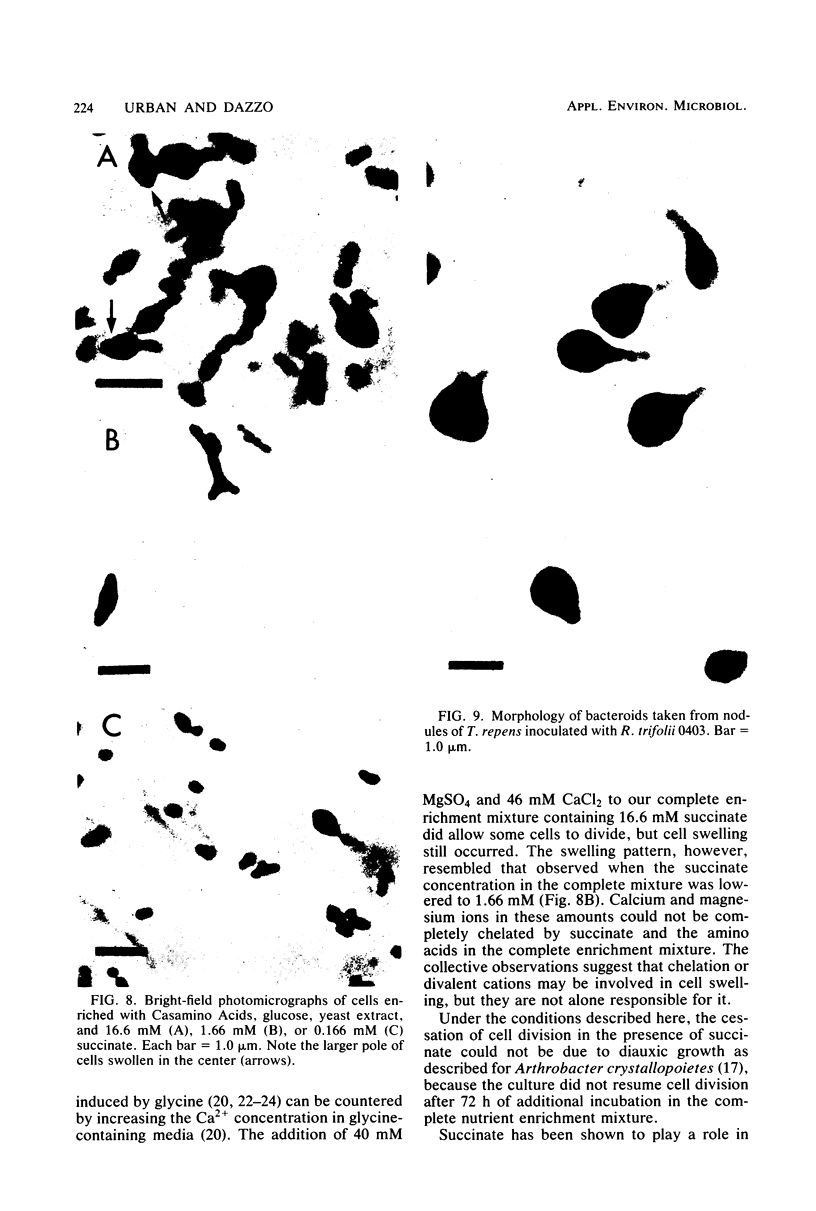
Morphological changes which accompany nutrient enrichment of Rhizobium trifolii 0403 were studied. Assays of cell number and size coupled with scanning electron microscopy and immunofluorescence microscopy showed that succinate induces cells to stop dividing in vitro and to swell either in the cell center or at one cell pole. The extent and frequency of in vitro cell swelling were in direct relation to the concentration of succinate added to the enrichment medium. The in vitro swelling of cells in 16.6 mM succinate plus Casamino Acids, glucose, and yeast extract closely resembled that of bacteroids of R. trifolii 0403 in nitrogen-fixing nodules of white clover. We hypothesize that succinate may be involved in the transformation of vegetative bacteria into the bacteroid morphology found in nitrogen-fixing nodules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B. Bacterial attachment as related to cellular recognition in the Rhizobium-legume symbiosis. J Supramol Struct Cell Biochem. 1981;16(1):29–41. doi: 10.1002/jsscb.1981.380160104. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Receptor site on clover and alfalfa roots for Rhizobium. Appl Environ Microbiol. 1977 Jan;33(1):132–136. doi: 10.1128/aem.33.1.132-136.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hrabak E. M. Presence of trifoliin A, a Rhizobium-binding lectin, in clover root exudate. J Supramol Struct Cell Biochem. 1981;16(2):133–138. doi: 10.1002/jsscb.1981.380160204. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Antigenic differences between infective and noninfective strains of Rhizobium trifolii. Appl Microbiol. 1975 Aug;30(2):172–177. doi: 10.1128/am.30.2.172-177.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Succinate transport in Rhizobium leguminosarum. J Bacteriol. 1981 Oct;148(1):193–202. doi: 10.1128/jb.148.1.193-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D. C. The bacteroids of the genus Rhizobium. Bacteriol Rev. 1962 Jun;26:119–141. [PMC free article] [PubMed] [Google Scholar]
- Jordan D. C., Coulter W. H. On the cytology and synthetic capacities of natural and artificially produced bacteroids of Rhizobium leguminosarum. Can J Microbiol. 1965 Aug;11(4):709–720. doi: 10.1139/m65-094. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Alteration of glucose metabolism of Arthrobacter crystallopoietes by compounds which induce sphere to rod morphogenesis. J Bacteriol. 1969 Feb;97(2):526–534. doi: 10.1128/jb.97.2.526-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Lyttleton P., Robertson J. G. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4284–4288. doi: 10.1073/pnas.78.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan J. B., Urban J. E. Growth response of Escherichia coli to nutritional shift-up: immediate division stimulation in slow-growing cells. J Bacteriol. 1976 Oct;128(1):302–308. doi: 10.1128/jb.128.1.302-308.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijdom B. W., Allen O. N. Medium supplementation with L- and D-amino acids relative to growth and efficiency of Rhizobium meliloti. Can J Microbiol. 1966 Apr;12(2):275–283. doi: 10.1139/m66-038. [DOI] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Organic Acid contents of soybean: age and source of nitrogen. Plant Physiol. 1981 Nov;68(5):989–991. doi: 10.1104/pp.68.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. E. Nondividing, Bacteroid-Like Rhizobium trifolii: In Vitro Induction Via Nutrient Enrichment. Appl Environ Microbiol. 1979 Dec;38(6):1173–1178. doi: 10.1128/aem.38.6.1173-1178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]