Abstract
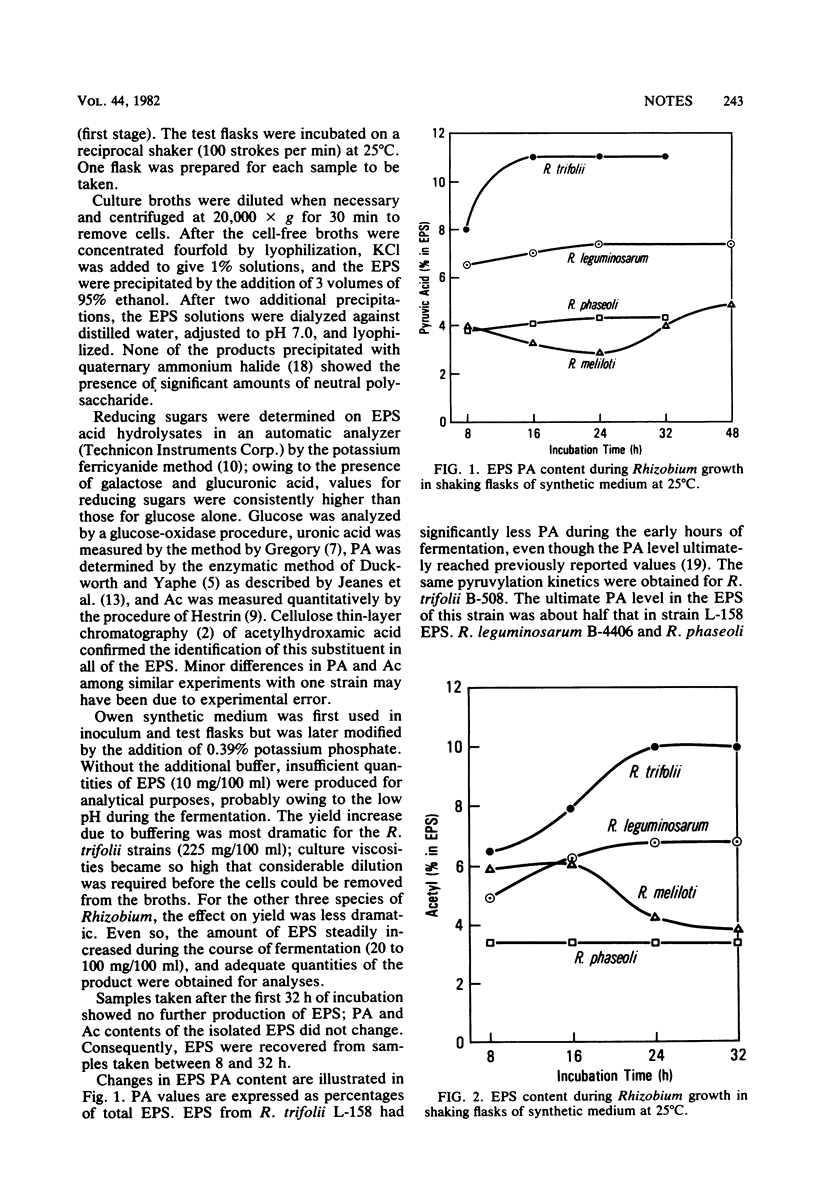
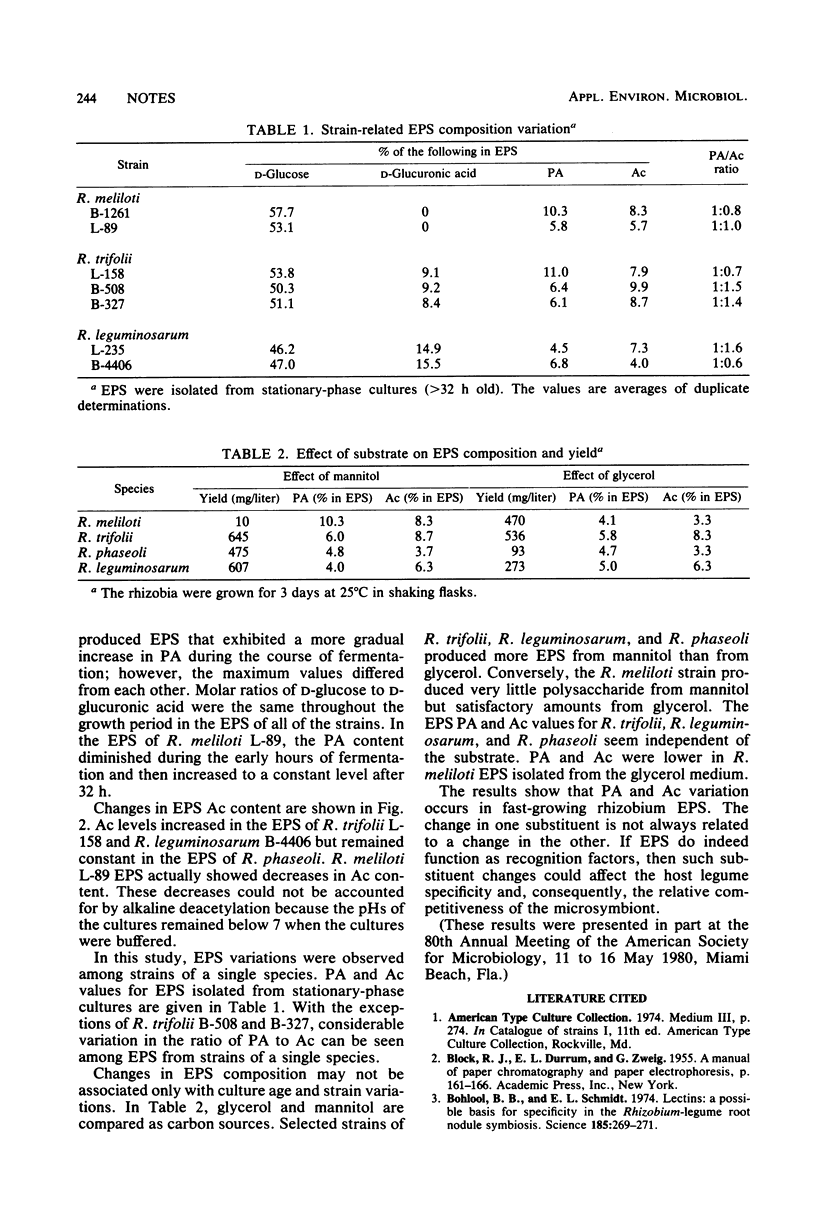
Pyruvic acid and O-acetyl groups are the major noncarbohydrate substituents in exopolysaccharides (EPS) produced by fast-growing species of Rhizobium. EPS substituent variations were observed among strains of the same species. The amounts of these substituents also varied with culture age; pyruvic acid increased in the EPS of all four species, whereas O-acetyl increased in Rhizobium trifolii and R. leguminosarum EPS, decreased in R. meliloti EPS, and remained constant in R. phaseoli EPS. The use of glycerol as a substrate for R. meliloti significantly increased EPS yields, whereas mannitol increased those of the other three Rhizobium species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Duckworth M., Yaphe W. Definitive assay for pyruvic acid in agar and other algal polysaccharides. Chem Ind. 1970 Jun 6;23:747–748. [PubMed] [Google Scholar]
- GREGORY J. D. The effect of borate on the carbazole reaction. Arch Biochem Biophys. 1960 Aug;89:157–159. doi: 10.1016/0003-9861(60)90036-9. [DOI] [PubMed] [Google Scholar]
- HUMPHREY B. A., VINCENT J. M. Extracellular polysaccharides of Rhizobium. J Gen Microbiol. 1959 Dec;21:477–484. doi: 10.1099/00221287-21-3-477. [DOI] [PubMed] [Google Scholar]
- Hepper C. M. Composition of extracellular polysaccharides of Rhizobium trifolii. Antonie Van Leeuwenhoek. 1972;38(3):437–445. doi: 10.1007/BF02328112. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Production of the Soybean-Chlorosis Toxin by Rhizobium japonicum in Pure Culture. Plant Physiol. 1965 Sep;40(5):931–933. doi: 10.1104/pp.40.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somme R. Chemical analysis of exocellular, acid polysaccharides from seven Rhizobium strains. Carbohydr Res. 1974 Mar;33(1):89–96. doi: 10.1016/s0008-6215(00)82942-0. [DOI] [PubMed] [Google Scholar]


