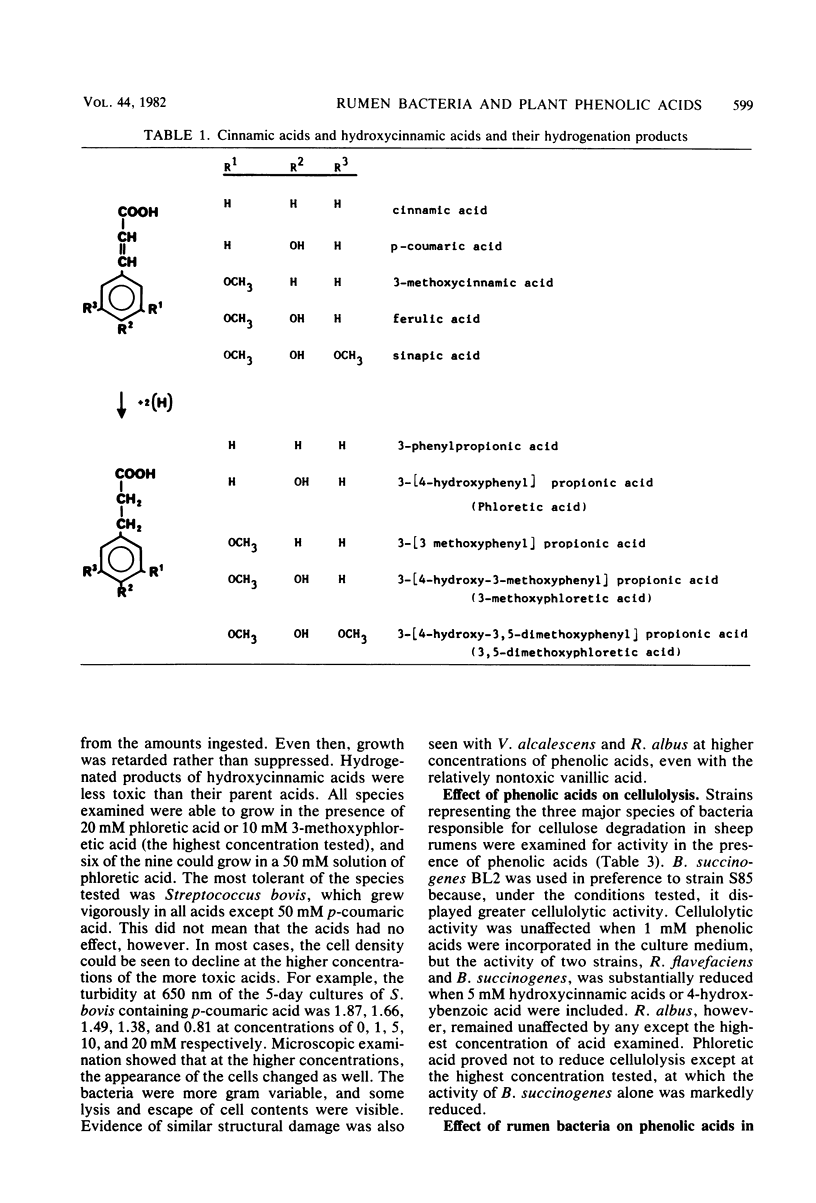
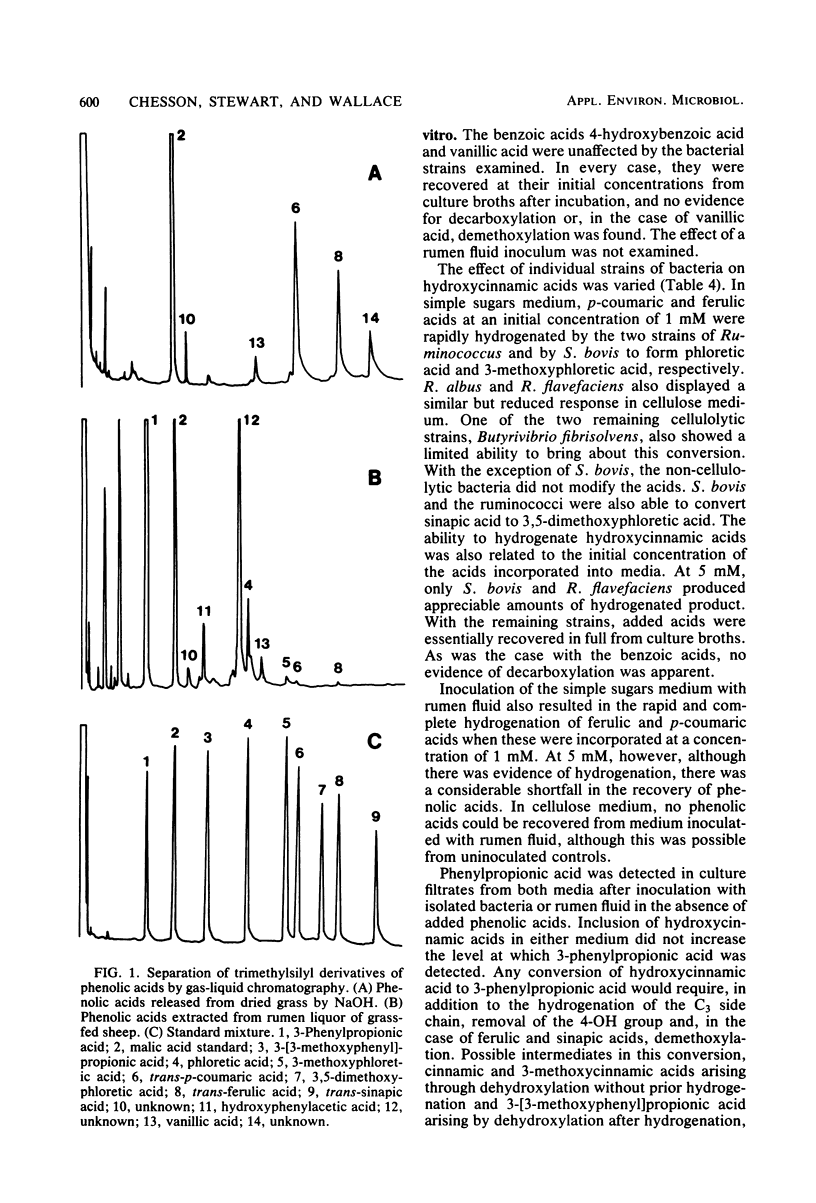
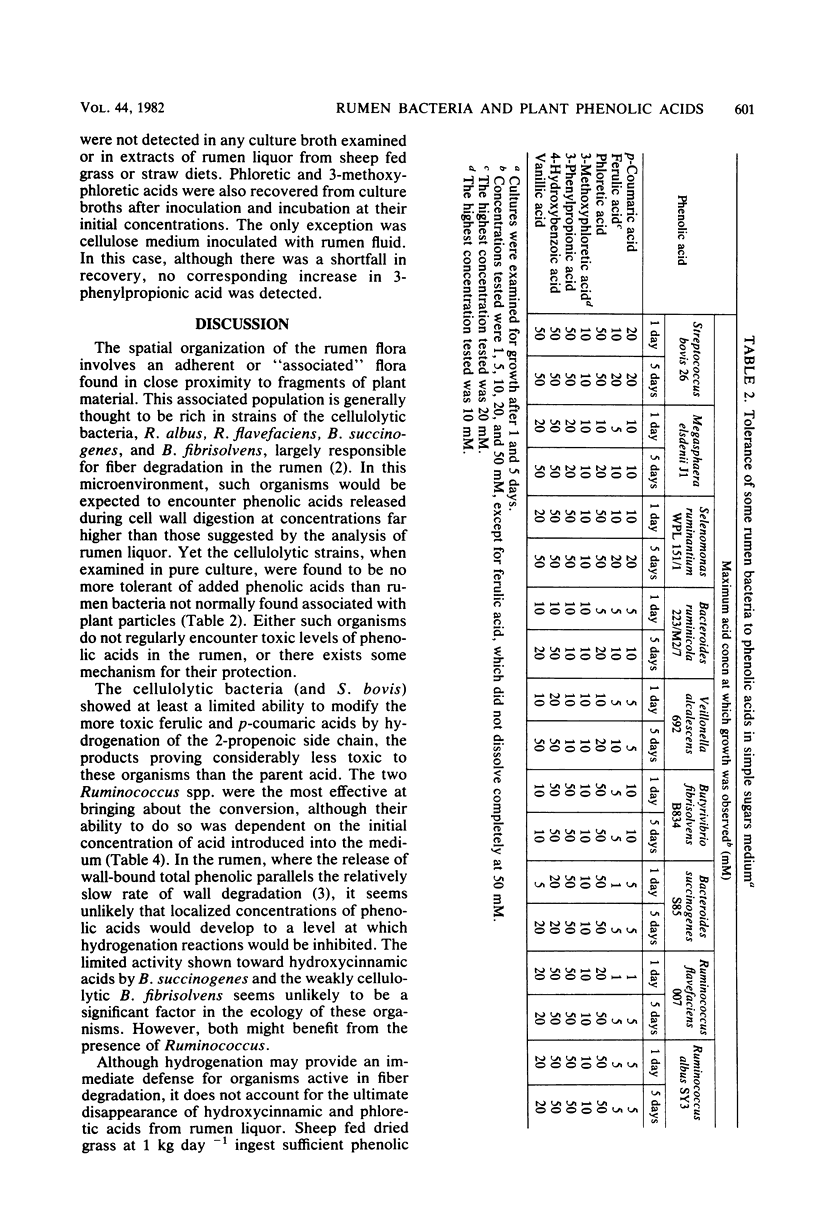
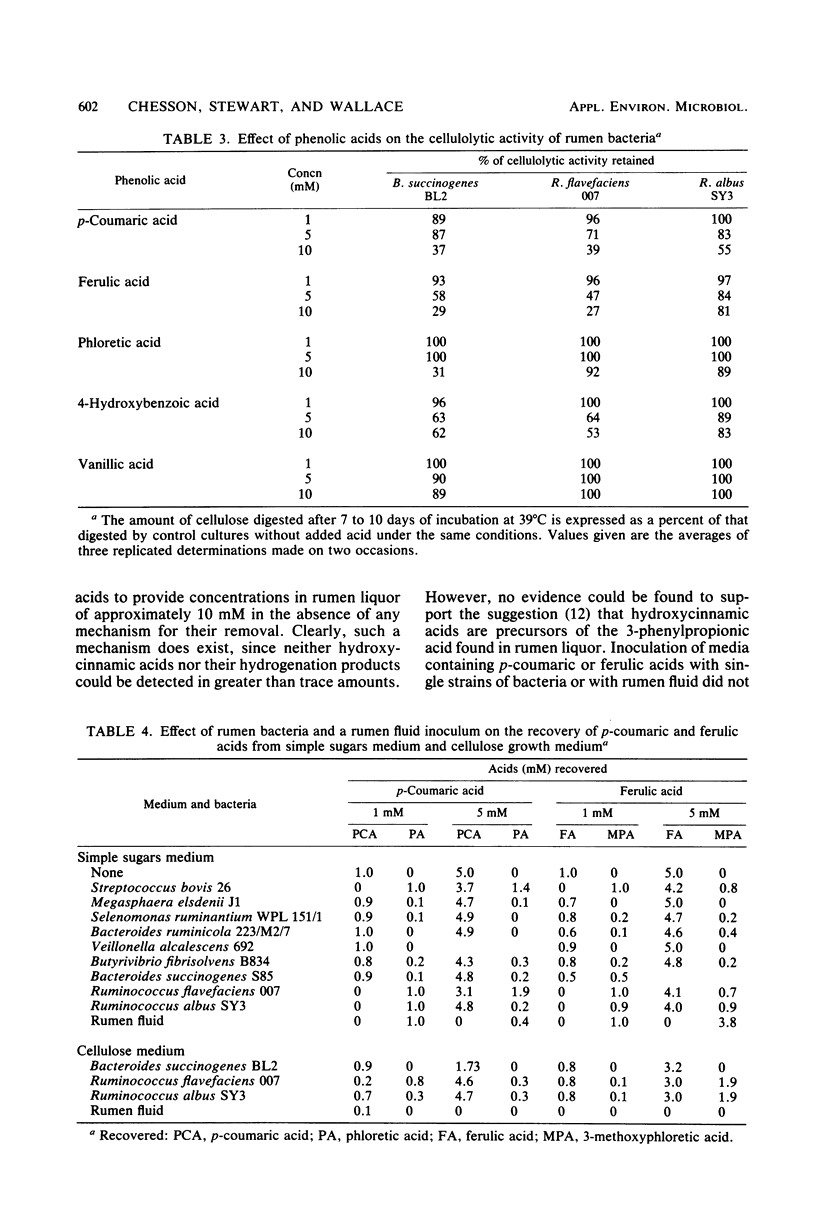
Abstract
Isolated rumen bacteria were examined for growth and, where appropriate, for their ability to degrade cellulose in the presence of the hydroxycinnamic acids trans-p-coumaric acid and trans-ferulic acid and the hydroxybenzoic acids vanillic acid and 4-hydroxybenzoic acid. Ferulic and p-coumaric acids proved to be the most toxic of the acids examined and suppressed the growth of the cellulolytic strains Ruminococcus albus, Ruminococcus flavefaciens, and Bacteroides succinogenes when included in a simple sugars medium at concentrations of >5 mM. The extent of cellulose digestion by R. flavefaciens and B. succinogenes but not R. albus was also substantially reduced. Examination of rumen fluid from sheep maintained on dried grass containing 0.51% phenolic acids showed the presence of phloretic acid (0.1 mM) and 3-methoxyphloretic acid (trace) produced by hydrogenation of the 2-propenoic side chain of p-coumaric and ferulic acids, respectively. The parent acids were found in trace amounts only, although they represented the major phenolic acids ingested. Phloretic and 3-methoxyphloretic acids proved to be considerably less toxic than their parent acids. All of the cellulolytic strains (and Streptococcus bovis) showed at least a limited ability to hydrogenate hydroxycinnamic acids, with Ruminococcus spp. proving the most effective. No further modification of hydroxycinnamic acids was produced by the single strains of bacteria examined. However, a considerable shortfall in the recovery of added phenolic acids was noted in media inoculated with rumen fluid. It is suggested that hydrogenation may serve to protect cellulolytic strains from hydroxycinnamic acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Attack on lignified grass cell walls by a facultatively anaerobic bacterium. Appl Environ Microbiol. 1980 Oct;40(4):809–820. doi: 10.1128/aem.40.4.809-820.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B., Holms W. H. Control of the sequential utilization of glucose and fructose by Escherichia coli. J Gen Microbiol. 1976 Aug;96(2):191–201. doi: 10.1099/00221287-95-2-191. [DOI] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979 Jul;38(1):84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Zemek J., Kosíková B., Augustín J., Joniak D. Antibiotic properties of lignin components. Folia Microbiol (Praha) 1979;24(6):483–486. doi: 10.1007/BF02927180. [DOI] [PubMed] [Google Scholar]


