Abstract
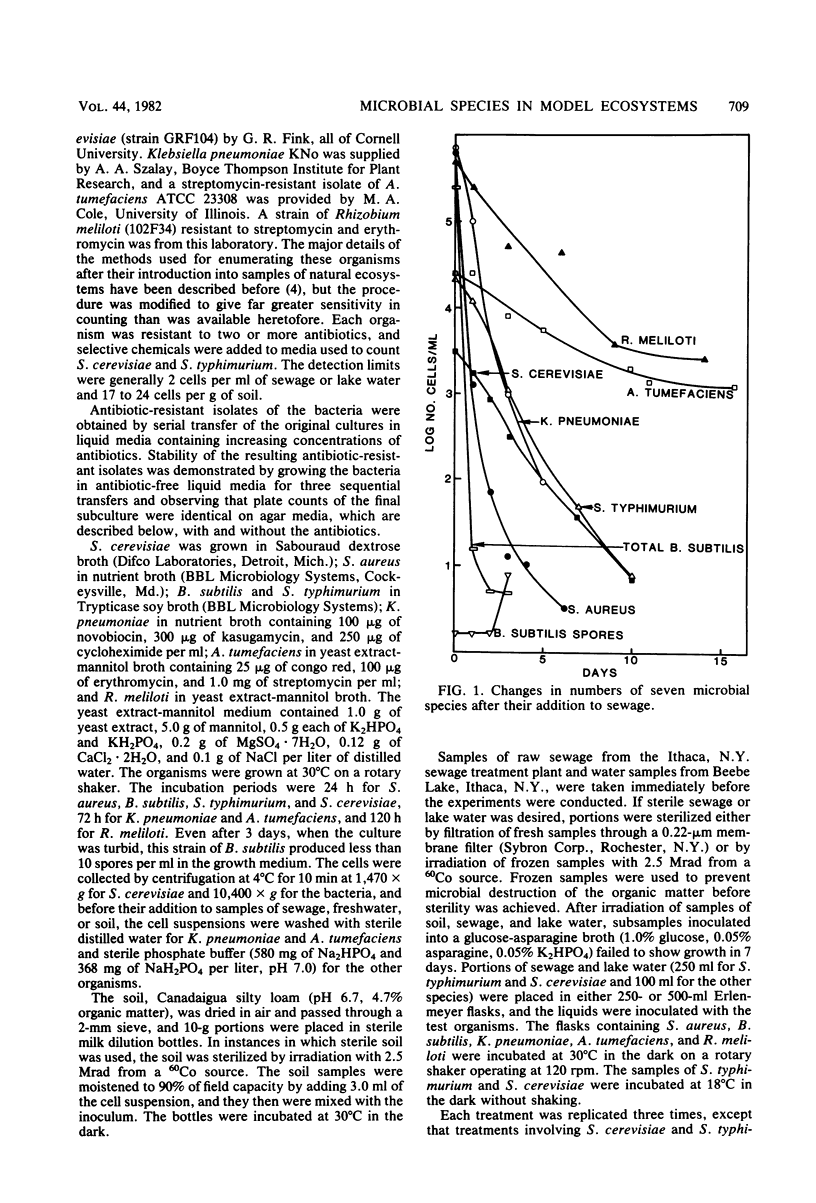
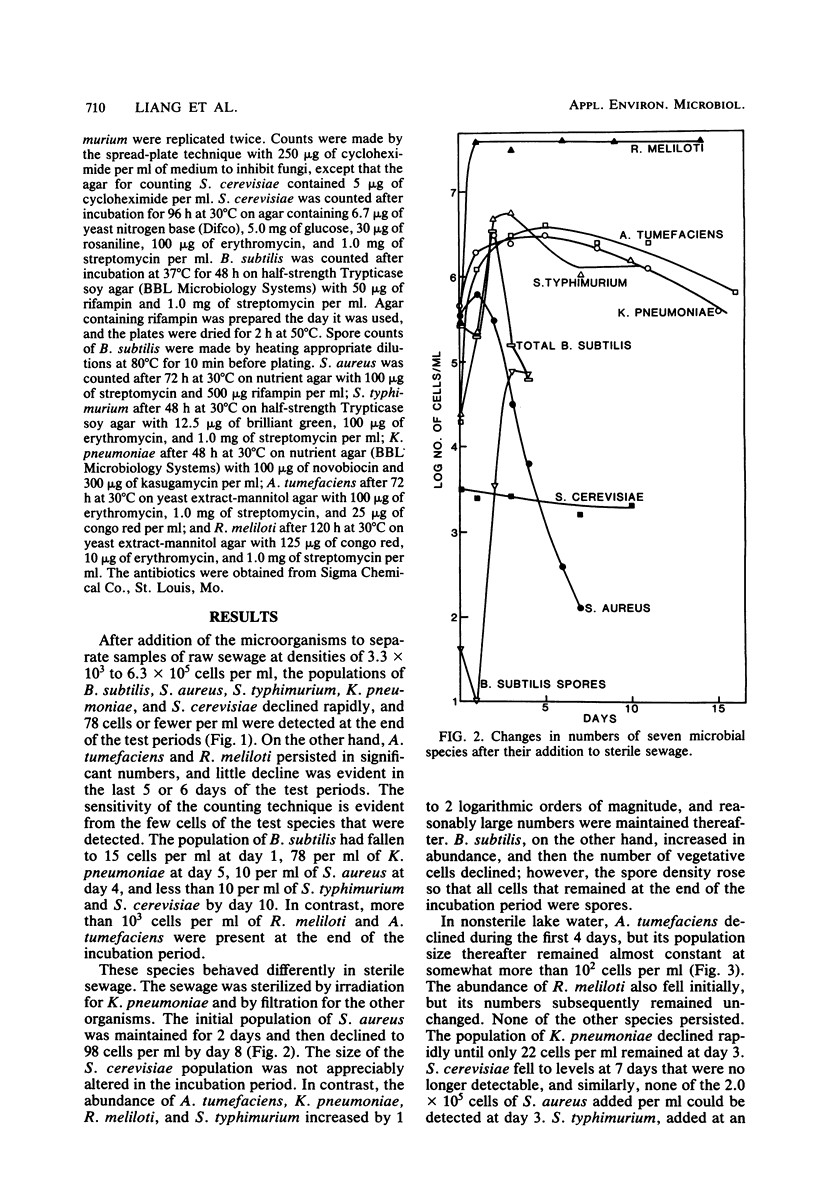
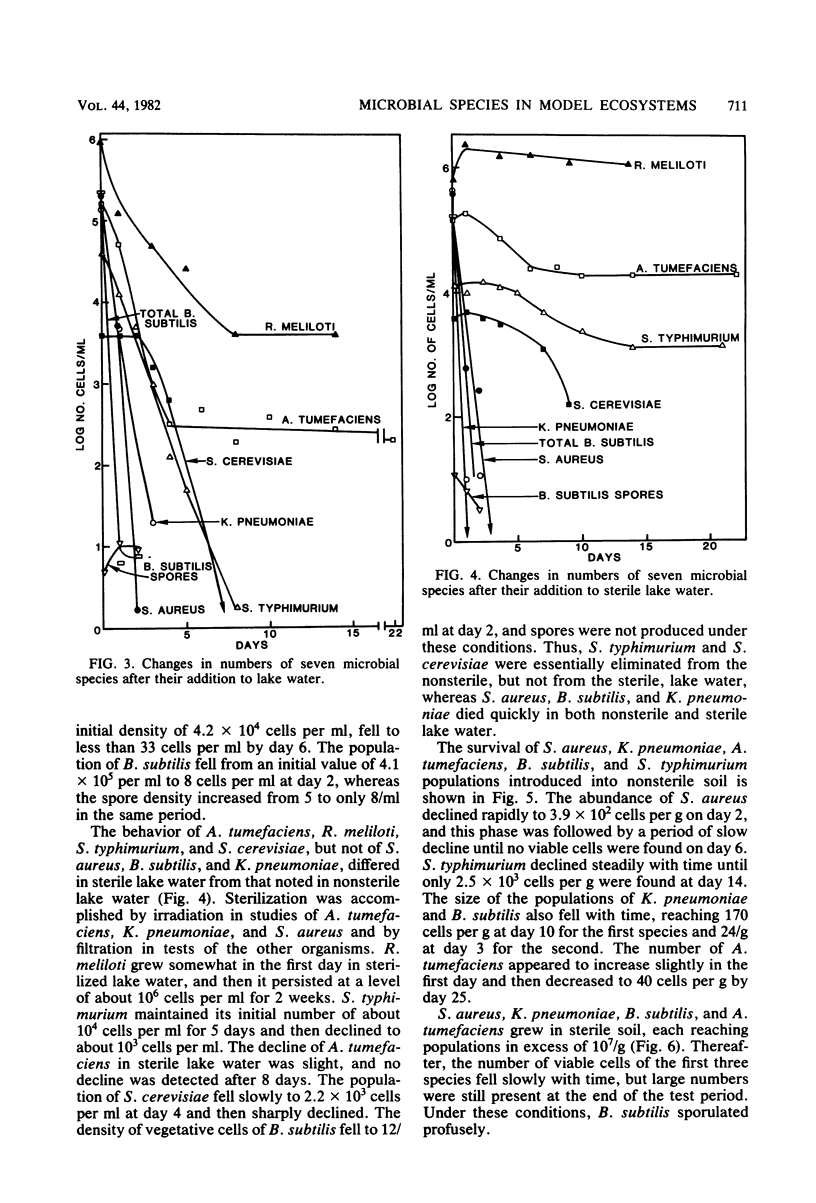
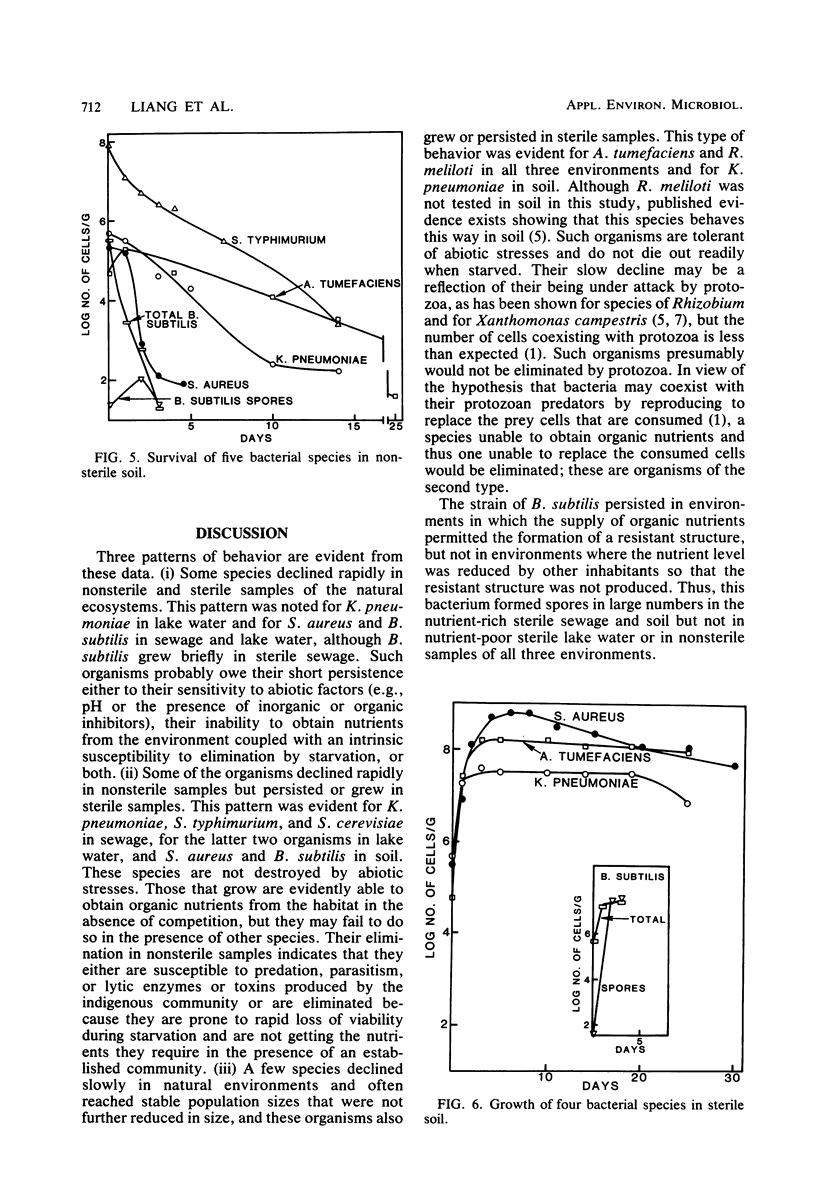
The changes in populations of Staphylococcus aureus, Bacillus subtilis, Salmonella typhimurium, Klebsiella pneumoniae, Agrobacterium tumefaciens, Rhizobium meliloti, and Saccharomyces cerevisiae were measured after their introduction into samples of sewage, lake water, and soil. Enumeration of small populations was possible because the strains used were resistant to antibiotics in concentrations and combinations such that few species native to these ecosystems were able to grow on agar containing the inhibitors. Fewer than 2 cells per ml of sewage or lake water and 25 cells per g of soil could be detected. A. tumefaciens and R. meliloti persisted in significant numbers with little decline, but S. aureus, K. pneumoniae, S. typhimurium, S. cerevisiae, and vegetative cells of B. subtilis failed to survive in samples of sewage and lake water. In sterile sewage, however, K. pneumoniae, B. subtilis, S. typhimurium, A. tumefaciens, and R. meliloti grew; S. cerevisiae populations were maintained at the levels used for inoculation; and S. aureus died rapidly. In sterile lake water, the population of S. aureus and K. pneumoniae and the number of vegetative cells of B. subtilis declined rapidly, R. meliloti grew, and the other species maintained significant numbers with little or a slow decline. The populations of S. aureus, K. pneumoniae, A. tumefaciens, B. subtilis, and S. typhimurium declined in soil, but the first four species grew in sterile soil. It is suggested that some species persist in environments in which they are not indigenous because they tolerate abiotic stresses, do not lose viability readily when starved, and coexist with antagonists. The species that fails to survive need only be affected by one of these factors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Why microbial predators and parasites do not eliminate their prey and hosts. Annu Rev Microbiol. 1981;35:113–133. doi: 10.1146/annurev.mi.35.100181.000553. [DOI] [PubMed] [Google Scholar]
- Conn H. J. Are Spore-forming Bacteria of any Significance in Soil under Normal Conditions? J Bacteriol. 1916 Mar;1(2):187–195. doi: 10.1128/jb.1.2.187-195.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso S. K., Habte M., Alexander M. Estimating the density of individual bacterial populations introduced into natural ecosytems. Can J Microbiol. 1973 Nov;19(11):1450–1451. doi: 10.1139/m73-234. [DOI] [PubMed] [Google Scholar]
- Danso S. K., Keya S. O., Alexander M. Protozoa and the decline of Rhizobium populations added to soil. Can J Microbiol. 1975 Jun;21(6):884–895. doi: 10.1139/m75-131. [DOI] [PubMed] [Google Scholar]
- Habte M., Alexander M. Further evidence for the regulation of bacterial populations in soil by protozoa. Arch Microbiol. 1977 Jun 20;113(3):181–183. doi: 10.1007/BF00492022. [DOI] [PubMed] [Google Scholar]
- Habte M., Alexander M. Protozoa as agents responsible for the decline of Xanthomonas campestris in soil. Appl Microbiol. 1975 Feb;29(2):159–164. doi: 10.1128/am.29.2.159-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Bissonnette G. K., Jezeski J. J., Thomson C. A., Stuart D. G. Comparative survival of indicator bacteria and enteric pathogens in well water. Appl Microbiol. 1974 May;27(5):823–829. doi: 10.1128/am.27.5.823-829.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means E. G., Olson B. H. Coliform inhibition by bacteriocin-like substances in drinking water distribution systems. Appl Environ Microbiol. 1981 Sep;42(3):506–512. doi: 10.1128/aem.42.3.506-512.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Sjogren R. E., Gibson M. J. Bacterial survival in a dilute environment. Appl Environ Microbiol. 1981 Jun;41(6):1331–1336. doi: 10.1128/aem.41.6.1331-1336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple K. L., Camper A. K., McFeters G. A. Survival of two enterobacteria in feces buried in soil under field conditions. Appl Environ Microbiol. 1980 Oct;40(4):794–797. doi: 10.1128/aem.40.4.794-797.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Zeigler B. P. Bacterial predator-prey interaction at low prey density. Appl Environ Microbiol. 1978 Jul;36(1):11–17. doi: 10.1128/aem.36.1.11-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


