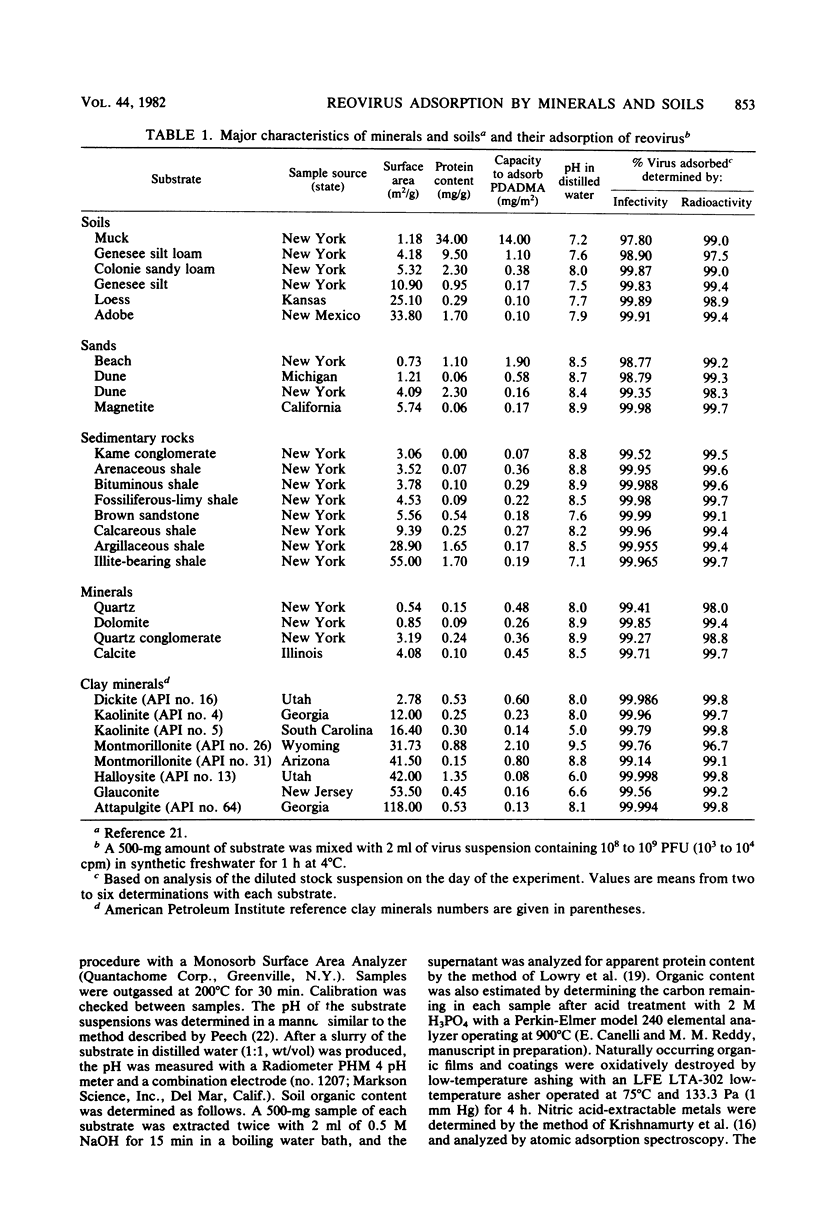
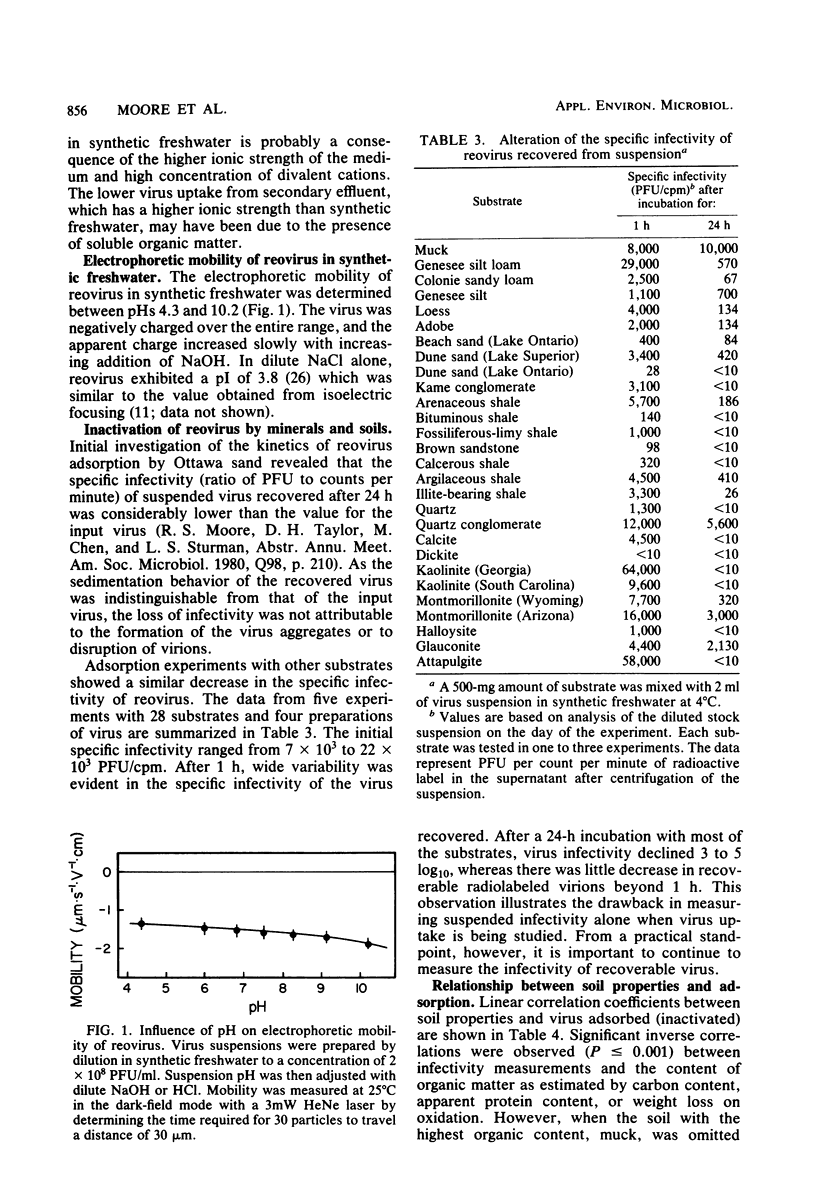
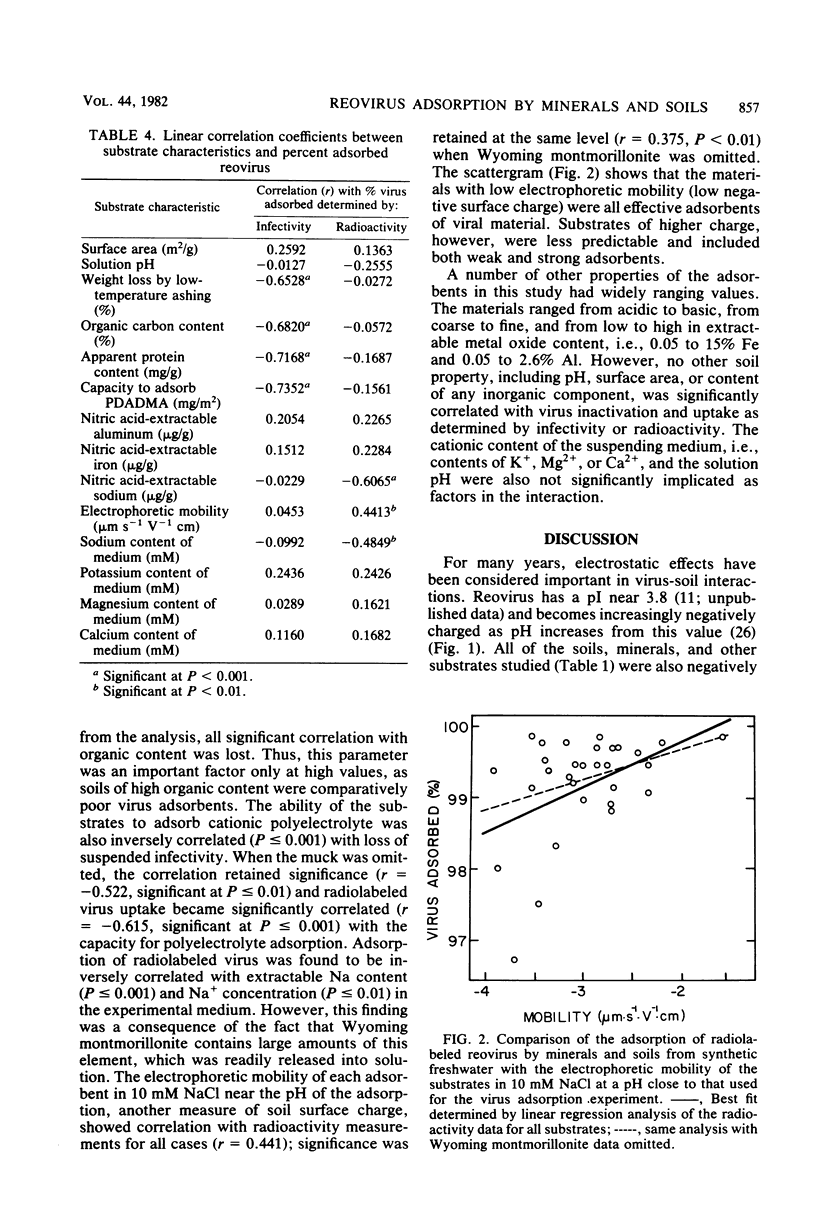
Abstract
Adsorption of [35S]methionine-labeled reovirus by 30 dry soils, minerals, and finely ground rocks suspended in synthetic freshwater at pH 7 was investigated to determine the conditions necessary for optimum virus removal during land application of wastewaters. All of the minerals and soils studied were excellent adsorbents of reovirus, with greater than 99% of the virus adsorbed after 1 h at 4 degrees C. Thereafter, virus remaining in suspension was significantly inactivated, and within 24 h a three to five log10 reduction in titer occurred. The presence of divalent cations, i.e., Ca2+ and Mg2+, in synthetic freshwater enhanced removal, whereas soluble organic matter decreased the amount of virus adsorbed in secondary effluent. The amount of virus adsorbed by these substrates was inversely correlated with the amount of organic matter, capacity to adsorb cationic polyelectrolyte, and electrophoretic mobility. Adsorption increased with increasing available surface area, as suspended infectivity was reduced further by the more finely divided substrates. However, the organic content of the soils reduced the level of infectious virus adsorbed below that expected from surface area measurements alone. The inverse correlation between virus adsorption and substrate capacity for cationic polyelectrolyte indicates that the adsorption of infectious reovirus particles is predominately a charged colloidal particle-charged surface interaction. Thus, adsorption of polyelectrolyte may be useful in predicting the fate of viruses during land application of sewage effluents and sludges.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedford A. J., Williams G., Bellamy A. R. Virus accumulation by the rock oyster Crassostrea glomerata. Appl Environ Microbiol. 1978 Jun;35(6):1012–1018. doi: 10.1128/aem.35.6.1012-1018.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboise S. M., Moore B. E., Sagik B. P. Poliovirus survival and movement in a sandy forest soil. Appl Environ Microbiol. 1976 Apr;31(4):536–543. doi: 10.1128/aem.31.4.536-543.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: effects of salts on the aggregation of poliovirus and reovirus at low pH. Appl Environ Microbiol. 1978 Jun;35(6):1084–1094. doi: 10.1128/aem.35.6.1084-1094.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979 Aug;38(2):241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lance J. C., Gerba C. P., Melnick J. L. Virus movement in soil columns flooded with secondary sewage effluent. Appl Environ Microbiol. 1976 Oct;32(4):520–526. doi: 10.1128/aem.32.4.520-526.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. S., Taylor D. H., Sturman L. S., Reddy M. M., Fuhs G. W. Poliovirus adsorption by 34 minerals and soils. Appl Environ Microbiol. 1981 Dec;42(6):963–975. doi: 10.1128/aem.42.6.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Dean C. H., Knuckles M. E., Wagner R. A. Interactions and survival of enteric viruses in soil materials. Appl Environ Microbiol. 1980 Jul;40(1):92–101. doi: 10.1128/aem.40.1.92-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. H., Bosmann H. B. Measurement of the electrokinetic properties of vaccinia and reovirus by laser-illuminated whole-particle microelectrophoresis. J Virol Methods. 1981 Apr;2(5):251–260. doi: 10.1016/0166-0934(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Taylor D. H., Moore R. S., Sturman L. S. Influence of pH and electrolyte composition on adsorption of poliovirus by soils and minerals. Appl Environ Microbiol. 1981 Dec;42(6):976–984. doi: 10.1128/aem.42.6.976-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W., Pierce L. V. Demonstration of virus in groundwater after effluent discharge onto soil. Appl Microbiol. 1975 Jun;29(6):751–757. doi: 10.1128/am.29.6.751-757.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


