Abstract
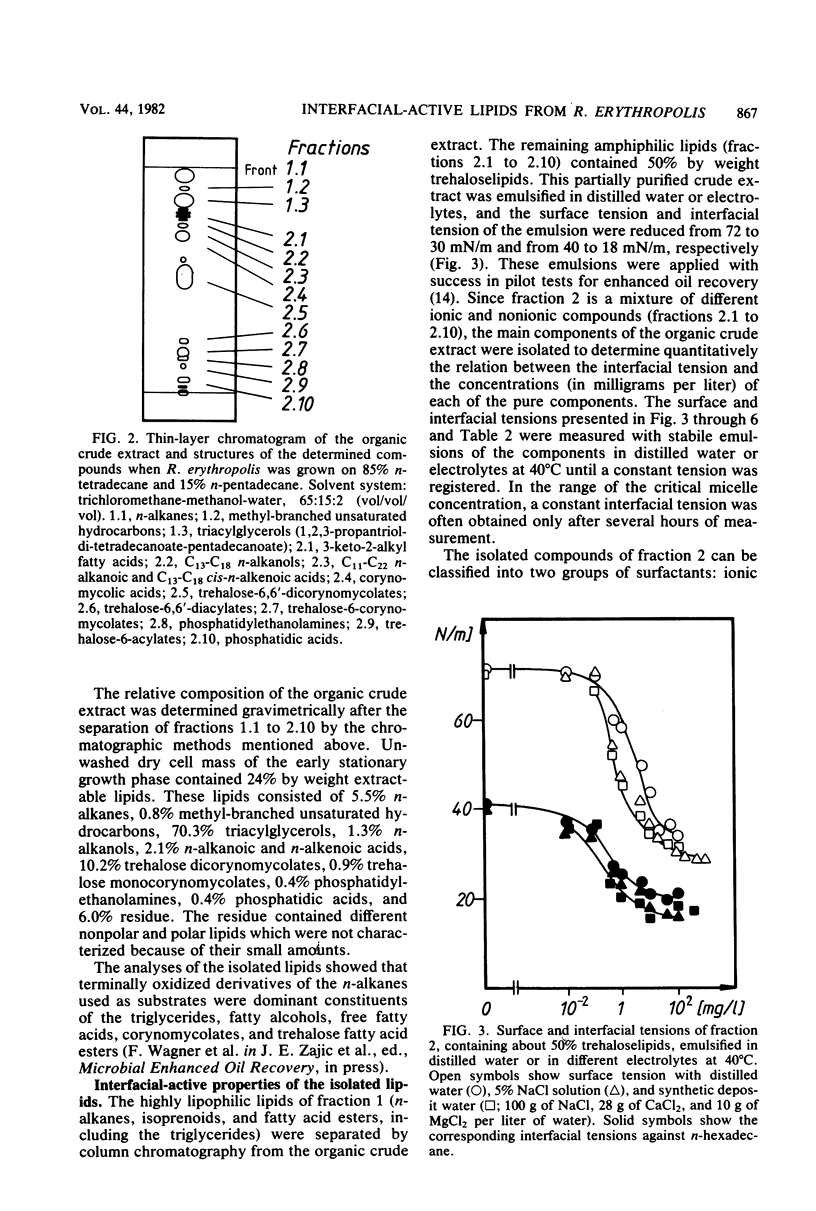
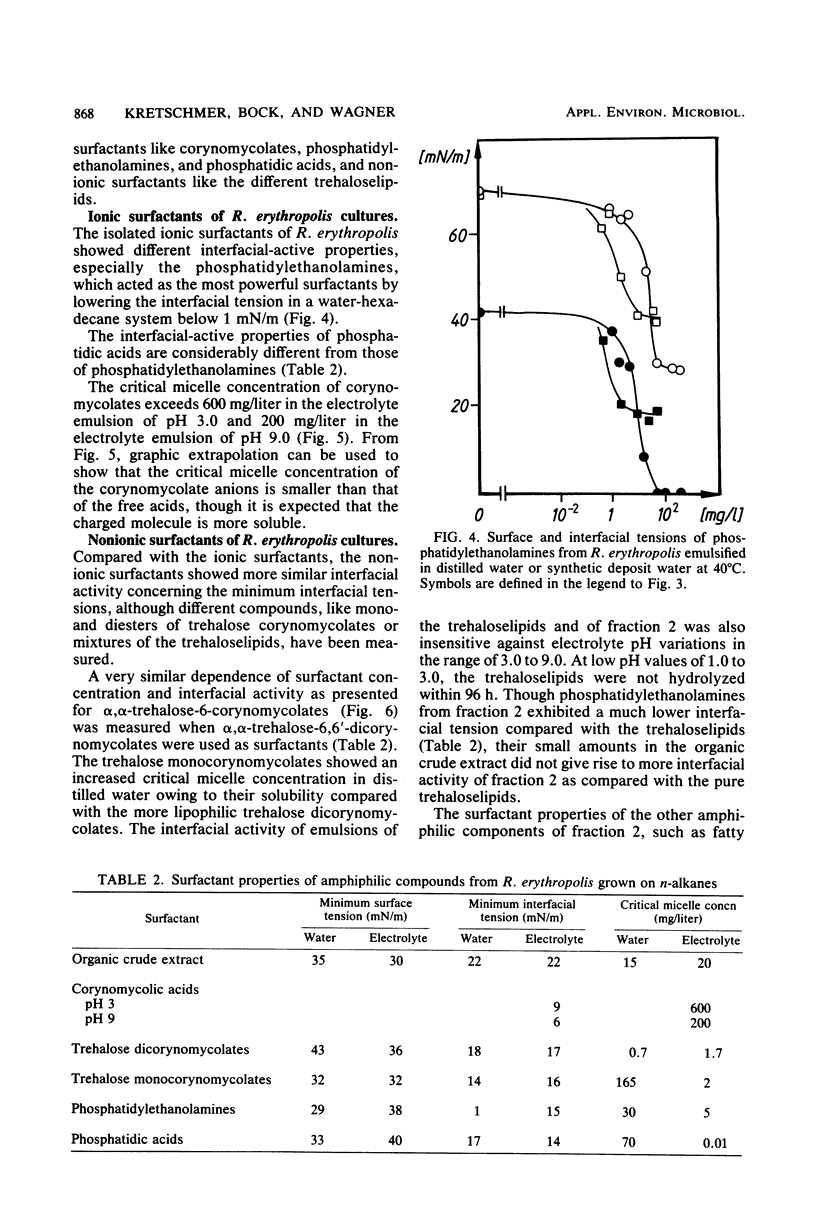
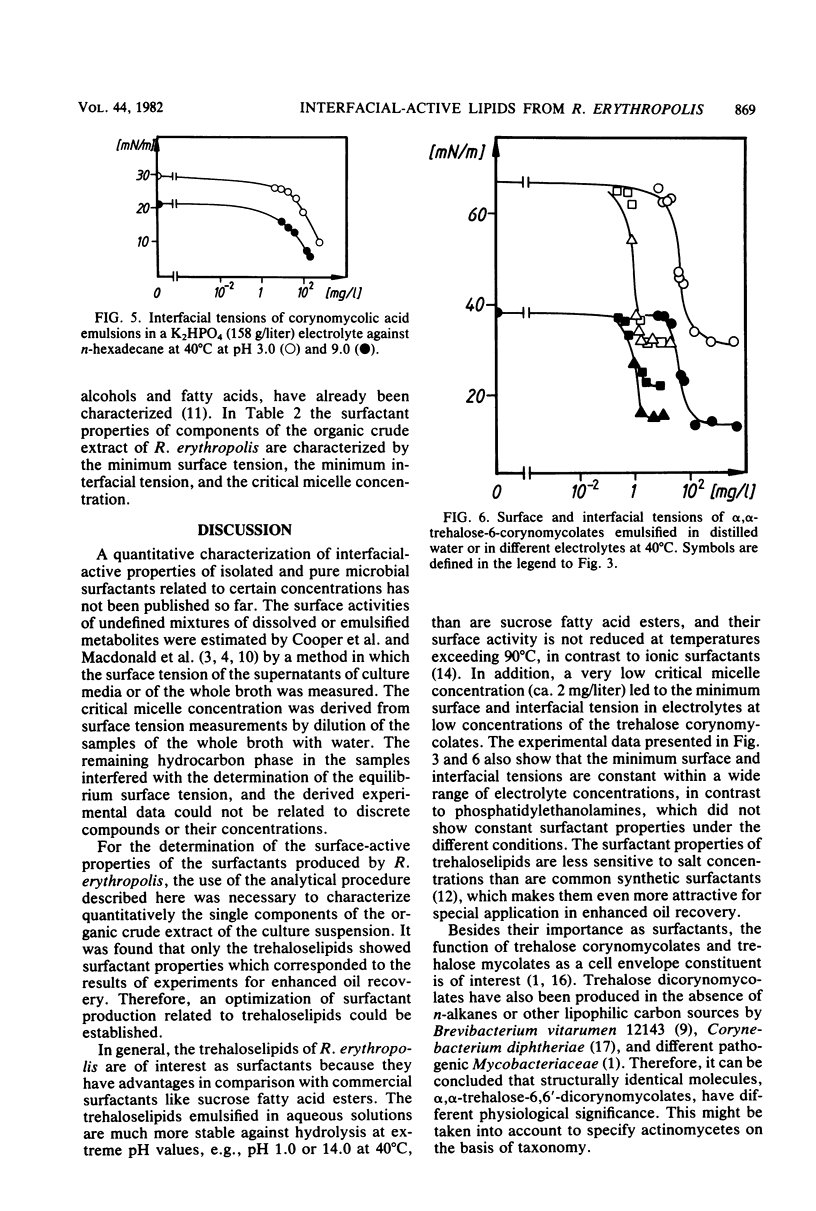
Lipophilic compounds of the culture suspension containing Rhodococcus erythropolis DSM43215 had surfactant properties when the bacteria were cultivated with n-alkanes as the sole carbon source. Thirteen main components from a dichloromethane-methanol extract of the R. erythropolis cultures were isolated and characterized to specify quantitatively their surfactant properties, e.g., minimum surface and interfacial tensions and critical micelle concentrations. The interfacial activity of the organic extract was dominated by α,α-trehalose-6,6′-dicorynomycolates which reduced interfacial tension from 44 to 18 mN/m. Phosphatidylethanolamines which were also present in the organic extract reduced the interfacial tension below 1 mN/m. The trehalose corynomycolates had extremely low critical micelle concentrations in high-salinity solutions, and the interfacial properties were stabile in solutions with a wide range of pH and ionic strength.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselineau C., Asselineau J. Trehalose-containing glycolipids. Prog Chem Fats Other Lipids. 1978;16:59–99. doi: 10.1016/0079-6832(78)90037-x. [DOI] [PubMed] [Google Scholar]
- Cooper D. G., Macdonald C. R., Duff S. J., Kosaric N. Enhanced Production of Surfactin from Bacillus subtilis by Continuous Product Removal and Metal Cation Additions. Appl Environ Microbiol. 1981 Sep;42(3):408–412. doi: 10.1128/aem.42.3.408-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. G., Zajic J. E., Gerson D. F. Production of surface-active lipids by Corynebacterium lepus. Appl Environ Microbiol. 1979 Jan;37(1):4–10. doi: 10.1128/aem.37.1.4-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanéelle M. A., Asselineau J. Glycolipids of brevibacterium vitarumen. Biochim Biophys Acta. 1976 Jan 18;486(1):205–208. doi: 10.1016/0005-2760(77)90085-6. [DOI] [PubMed] [Google Scholar]
- Macdonald C. R., Cooper D. G., Zajic J. E. Surface-Active Lipids from Nocardia erythropolis Grown on Hydrocarbons. Appl Environ Microbiol. 1981 Jan;41(1):117–123. doi: 10.1128/aem.41.1.117-123.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzinger G. S., Meredith S. C., Takayama K., Hunter R. L., Kézdy F. J. The role of surface in the biological activities of trehalose 6,6'-dimycolate. Surface properties and development of a model system. J Biol Chem. 1981 Aug 10;256(15):8208–8216. [PubMed] [Google Scholar]
- Senn M., Ioneda T., Pudles J., Lederer E. Spectrométrie de masse de glycolipides. I. Structure du "cord factor" de Corynebacterium diphtheriae. Eur J Biochem. 1967 May;1(3):353–356. doi: 10.1111/j.1432-1033.1967.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Wong M. Y., Gray G. R. Structures of the homologous series of monoalkene mycolic acids from Mycobacterium smegmatis. J Biol Chem. 1979 Jul 10;254(13):5741–5744. [PubMed] [Google Scholar]
- Wong M. Y., Steck P. A., Gray G. R. The major mycolic acids of Mycobacterium smegmatis. Characterization of their homologous series. J Biol Chem. 1979 Jul 10;254(13):5734–5740. [PubMed] [Google Scholar]


