Abstract
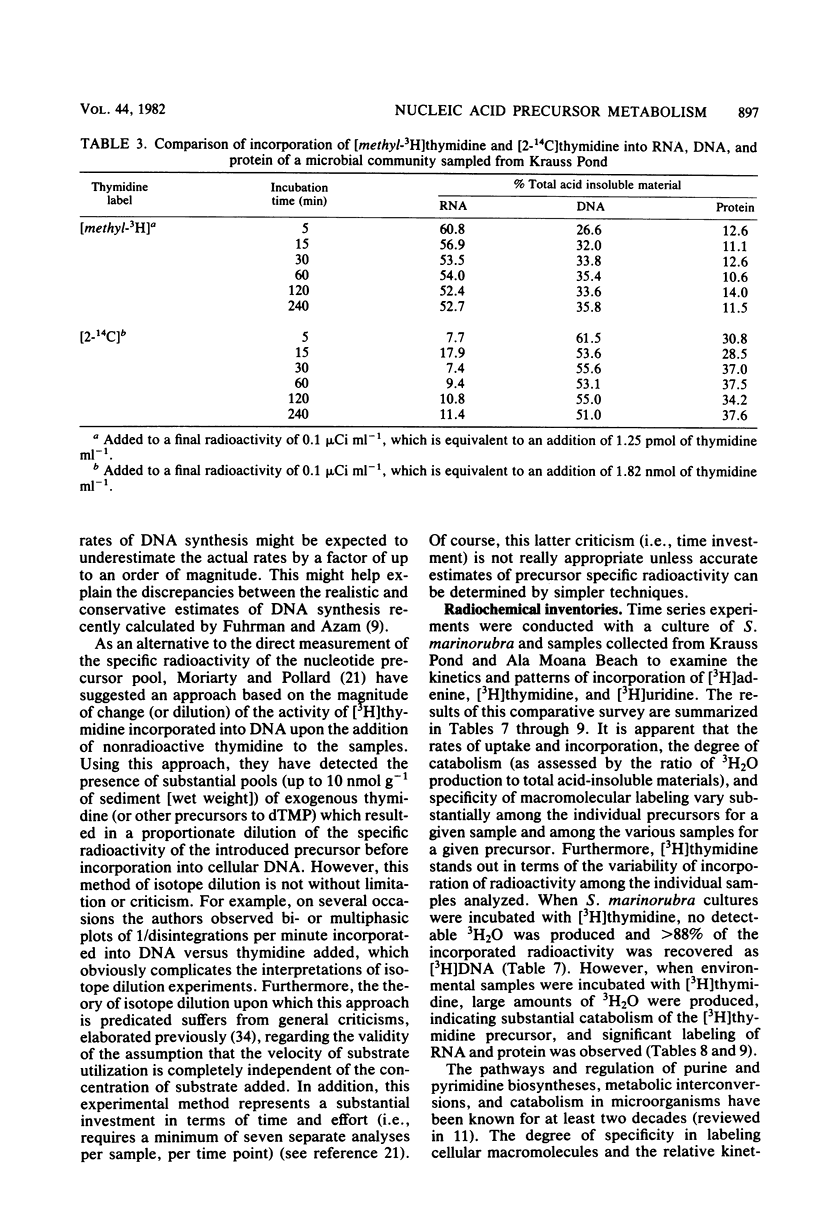
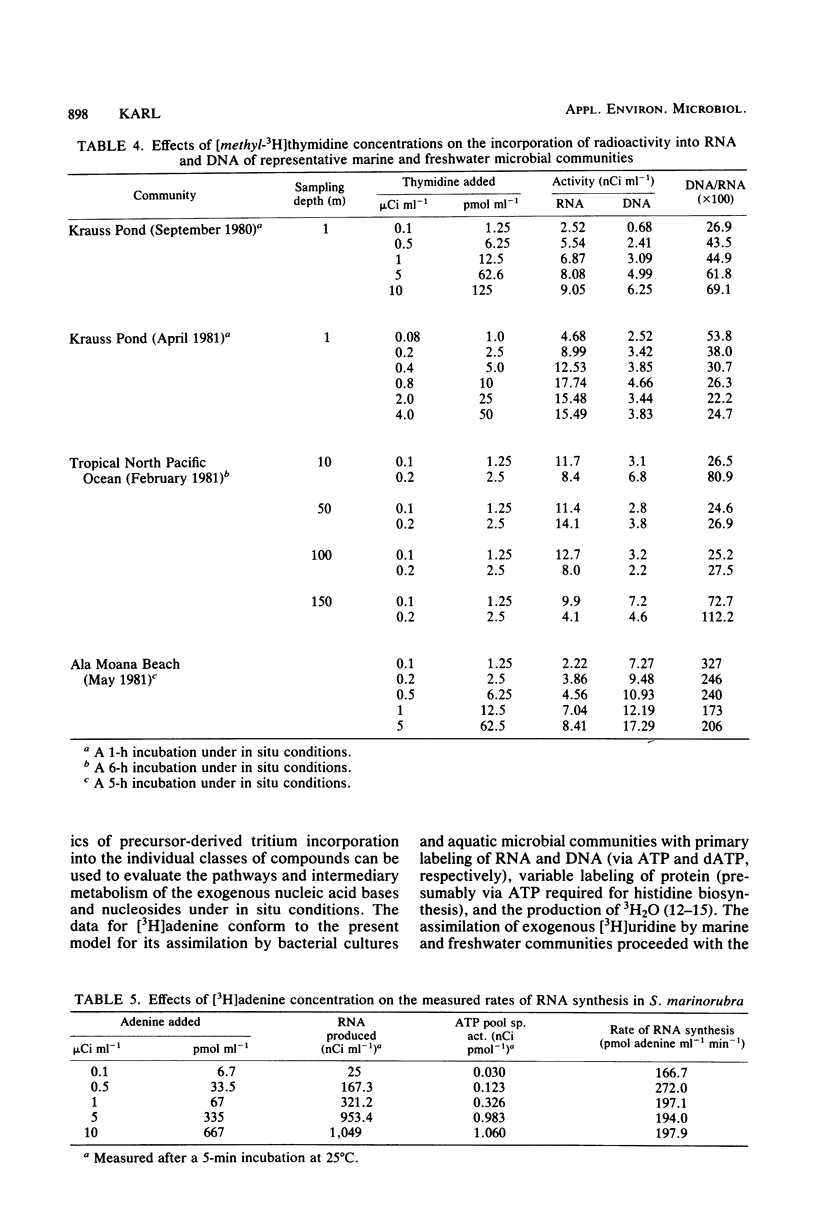
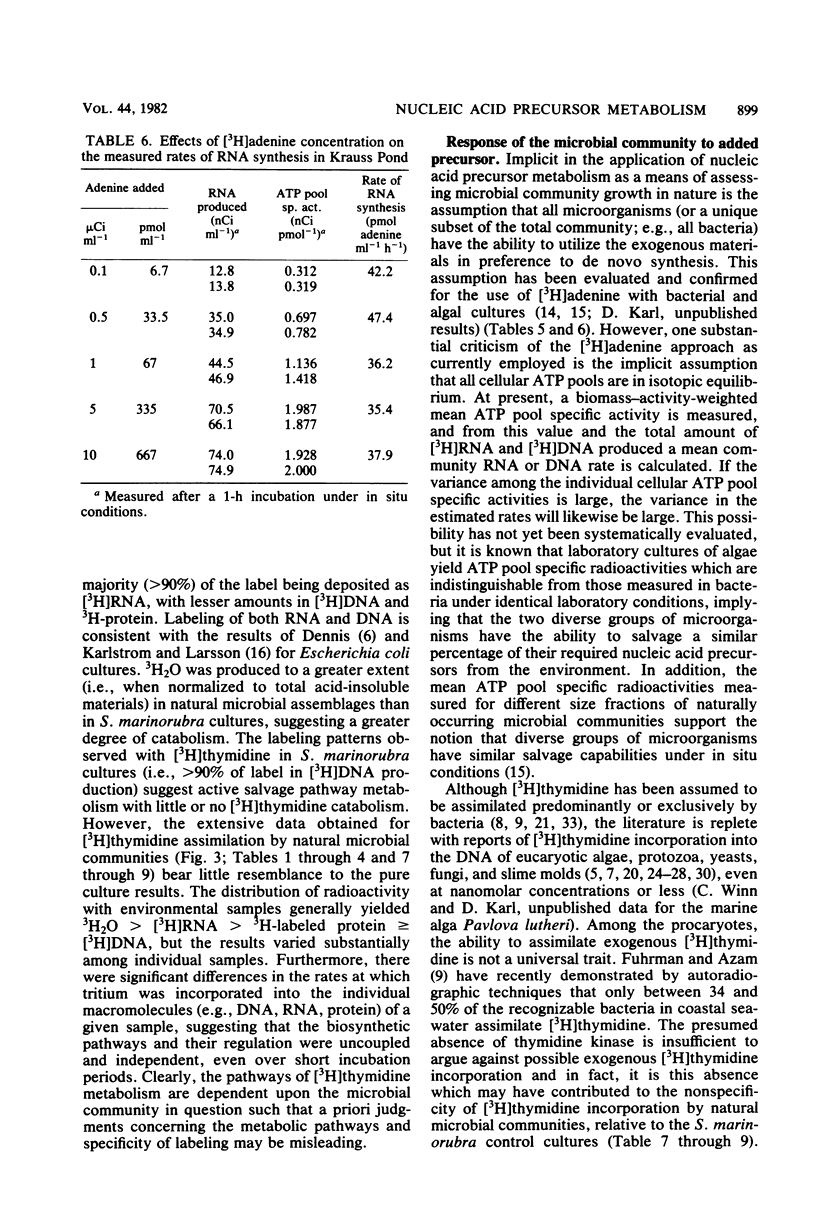
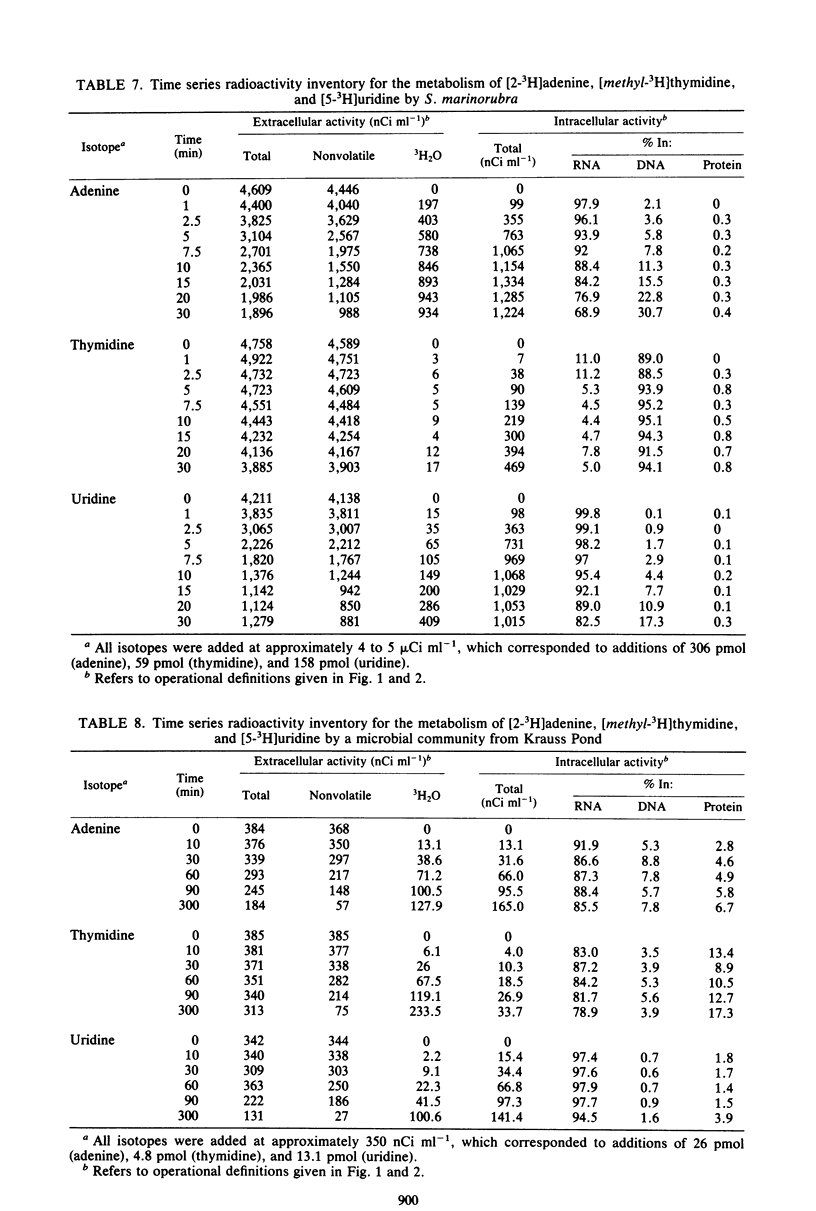
The use of radiolabeled nucleosides and nucleic acid bases to estimate the rates of RNA and DNA synthesis in naturally occurring microbial assemblages requires numerous assumptions, several of which are evaluated herein. Comparative time series analyses of the uptake and incorporation, labeling specificity, and extent of catabolism of [2-3H]adenine, [methyl-3H]thymidine, and [5-3H]uridine were performed with pure bacterial and algal cultures, as well as with environmental samples. [3H]thymidine yielded the most variable results, especially with regard to the extent of nonspecific macromolecular labeling. The pathways of [3H]thymidine and [3H]adenine metabolism were further evaluated by isotope dilution methods and by comparing incorporation patterns of thymidine labeled at different sites of the molecule. The advantages, uncertainties, and limitations of the use of radiolabeled nucleic acid precursors in studies of aquatic microbial ecology are discussed and a prospectus for future studies presented.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azam F., Beers J. R., Campbell L., Carlucci A. F., Holm-Hansen O., Reid F. M., Karl D. M. Occurrence and metabolic activity of organisms under the ross ice shelf, antarctica, at station j9. Science. 1979 Feb 2;203(4379):451–453. doi: 10.1126/science.203.4379.451. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Bacterial growth rate in the sea: direct analysis by thymidine autoradiography. Science. 1967 Jan 6;155(3758):81–83. doi: 10.1126/science.155.3758.81. [DOI] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. Factors influencing the plate method for determining abundance of bacteria in sea water. Proc Soc Exp Biol Med. 1957 Nov;96(2):392–394. doi: 10.3181/00379727-96-23487. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Regulation of ribosomal and transfer ribonucleic acid synthesis in Escherichia coli B-r. J Biol Chem. 1972 May 10;247(9):2842–2845. [PubMed] [Google Scholar]
- FINK R. M., FINK K. Relative retention of H3 and C14 labels of nucleosides incorporated into nucleic acids of Neurospora. J Biol Chem. 1962 Sep;237:2889–2891. [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Karl D. M. Measurement of microbial activity and growth in the ocean by rates of stable ribonucleic Acid synthesis. Appl Environ Microbiol. 1979 Nov;38(5):850–860. doi: 10.1128/aem.38.5.850-860.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Simultaneous rates of ribonucleic Acid and deoxyribonucleic Acid syntheses for estimating growth and cell division of aquatic microbial communities. Appl Environ Microbiol. 1981 Nov;42(5):802–810. doi: 10.1128/aem.42.5.802-810.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlström O., Larsson A. Significance of ribonucleotide reduction in the biosynthesis of deoxyribonucleotides in Escherichia coli. Eur J Biochem. 1967 Dec;3(2):164–170. doi: 10.1111/j.1432-1033.1967.tb19512.x. [DOI] [PubMed] [Google Scholar]
- Larock P. A., Lauer R. D., Schwarz J. R., Watanabe K. K., Wiesenburg D. A. Microbial biomass and activity distribution in an anoxic, hypersaline basin. Appl Environ Microbiol. 1979 Mar;37(3):466–470. doi: 10.1128/aem.37.3.466-470.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michrina C. A., Deering R. A. Thymidine kinase activity in Dictyostelium discoideum. J Gen Microbiol. 1980 Jul;119(1):263–266. doi: 10.1099/00221287-119-1-263. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth, RNA, and protein synthesis. Annu Rev Microbiol. 1978;32:393–432. doi: 10.1146/annurev.mi.32.100178.002141. [DOI] [PubMed] [Google Scholar]
- PLAUT W., SAGAN L. A. Incorporation of thymidine in the cytoplasm of Amoeba proteus. J Biophys Biochem Cytol. 1958 Nov 25;4(6):843–846. doi: 10.1083/jcb.4.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGAN L. AN UNUSUAL PATTERN OF TRITIATED THYMIDINE INCORPORATION IN EUGLENA. J Protozool. 1965 Feb;12:105–109. doi: 10.1111/j.1550-7408.1965.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Steffensen D. M., Sheridan W. F. Incorporation of H3-thymidine into chloroplast DNA of marine algae. J Cell Biol. 1965 Jun;25(3):619–626. doi: 10.1083/jcb.25.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straat P. A., Wolochow H., Dimmick R. L., Chatigny M. A. Evidence for incorporation of thymidine into deoxyribonucleic acid in airborne bacterial cells. Appl Environ Microbiol. 1977 Sep;34(3):292–296. doi: 10.1128/aem.34.3.292-296.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinton D. C., Hanawalt P. C. In vivo specific labeling of Chlamydomonas chloroplast DNA. J Cell Biol. 1972 Sep;54(3):592–597. doi: 10.1083/jcb.54.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]


