Abstract
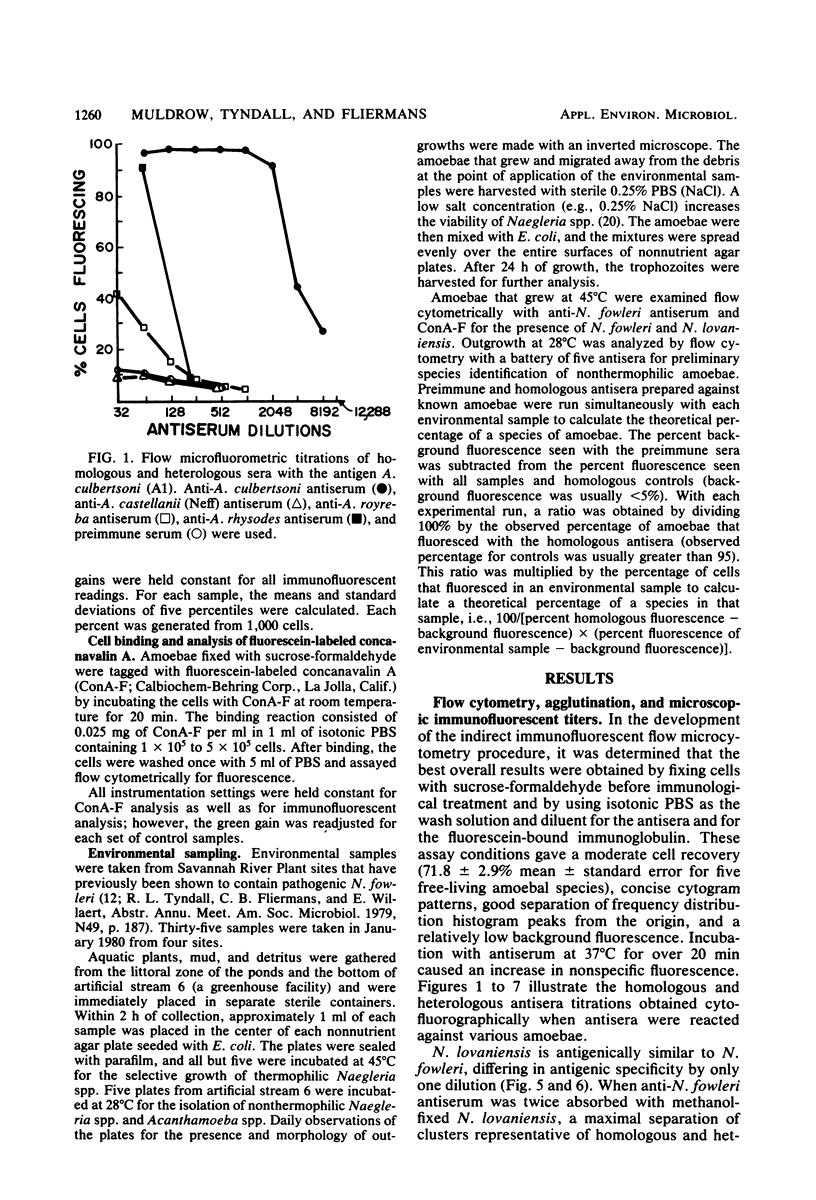
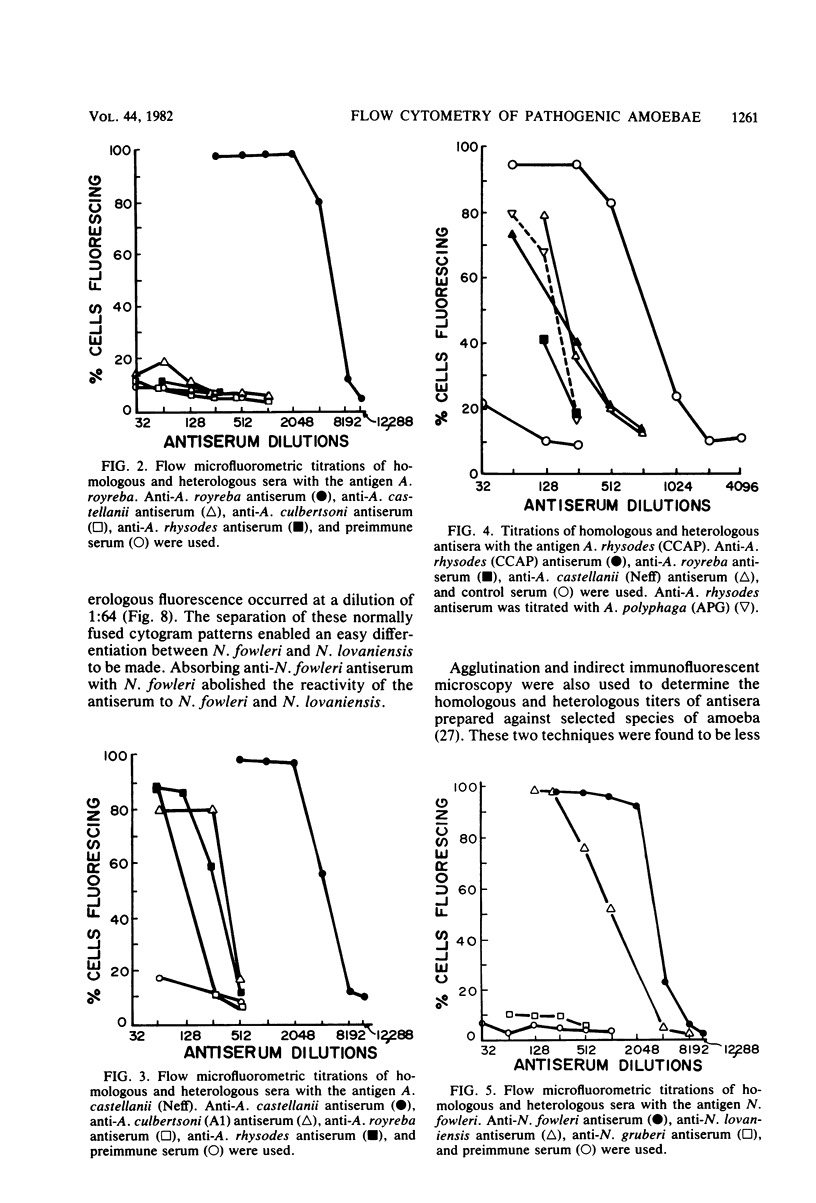
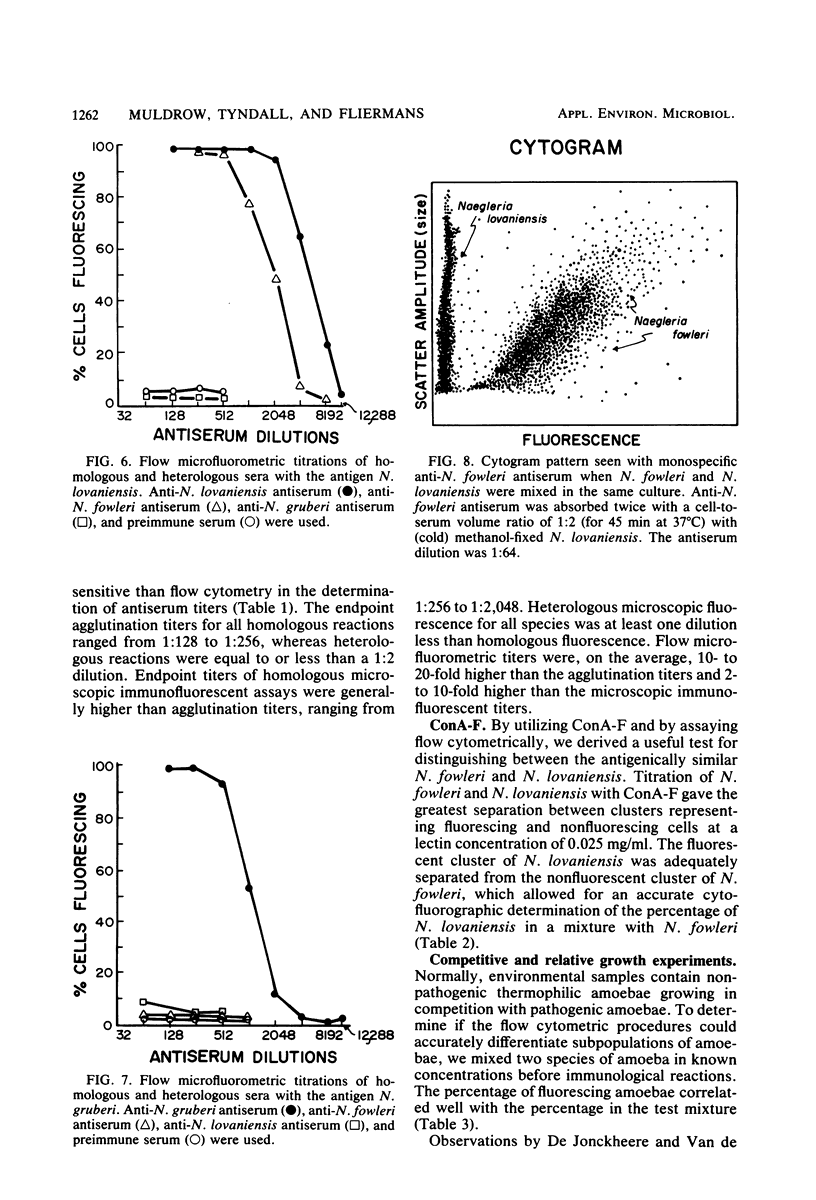
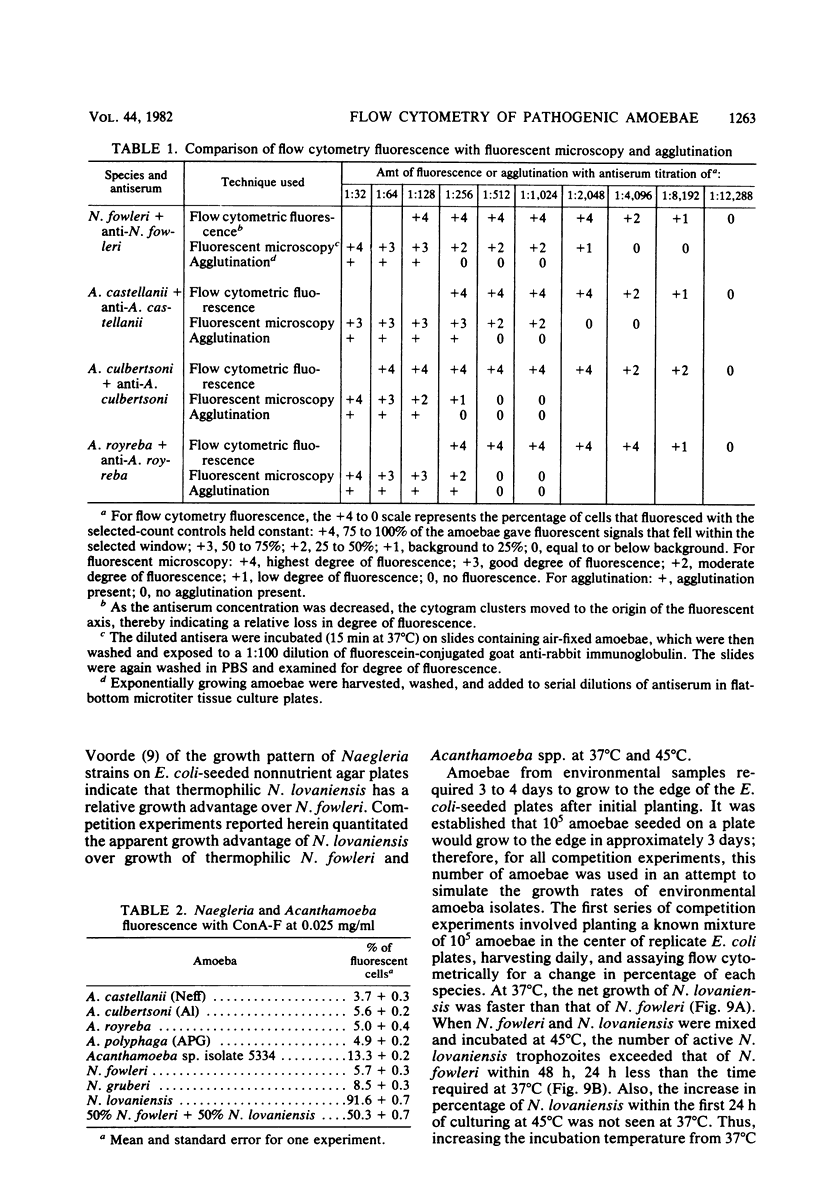
Species of small, free-living amoebae of the genera Naegleria and Acanthamoeba can cause fatal amoebic meningoencephalitis. Previous investigations have shown that pathogenic amoebae are associated with thermally altered water. Flow cytometric techniques for identifying species of pathogenic and nonpathogenic amoebae from such water have been developed, using immunofluorescence and fluorescein-bound concanavalin A. Flow cytometry is accomplished with a cytofluorograph, in which cells are dispersed in a suspended carrier liquid and passed in front of a focused argon ion laser beam. Cells are then distinguished by the degree of scattered light (size) or fluorescence. Flow cytometry techniques have proven efficient for environmental samples, as indicated by the identification of pathogenic Naegleria fowleri and nonpathogenic Naegleri gruberi and Acanthamoeba castellanii isolated from the Savannah River Plant in South Carolina. Cytofluorographic analysis of environmental samples has several advantages over the current methods of isolation and classification of free-living amoebae. With this system, it is possible to rapidly identify species and quantitate mixtures of pathogenic amoebae in environmental samples. Cytofluorographic analysis of amoebic isolates reduces the time presently required to screen environmental sites for pathogenic amoebae. The cytofluorograph permits detection and species identification of nonthermophilic Naegleria spp. and Acanthamoeba spp. that could not easily be isolated for species identification by conventional methods. Other advantages of flow cytometry over fluorescent microscopy include a high degree of statistical precision due to the large numbers measured, high immunofluorescent titers, and elimination of subjectivity and fluorescence fading.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972 Aug 19;2(7773):379–379. doi: 10.1016/s0140-6736(72)91763-1. [DOI] [PubMed] [Google Scholar]
- Bhagwandeen S. B., Carter R. F., Naik K. G., Levitt D. A case of hartmannellid amebic meningoencephalitis in Zambia. Am J Clin Pathol. 1975 Apr;63(4):483–492. doi: 10.1093/ajcp/63.4.483. [DOI] [PubMed] [Google Scholar]
- CHANG S. L. Cultural, cytological and ecological observations on the amoeba stage of Naegleria gruberi. J Gen Microbiol. 1958 Jun;18(3):565–578. doi: 10.1099/00221287-18-3-565. [DOI] [PubMed] [Google Scholar]
- CULBERTSON C. G., SMITH J. W., MINNER J. R. Acanthamoeba: observations on animal pathogenicity. Science. 1958 Jun 27;127(3313):1506–1506. doi: 10.1126/science.127.3313.1506. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Primary amoebic meningo-encephalitis: clinical, pathological and epidemiological features of six fatal cases. J Pathol Bacteriol. 1968 Jul;96(1):1–25. doi: 10.1002/path.1700960102. [DOI] [PubMed] [Google Scholar]
- Coulson P. B., Tyndall R. Quantation by flow microfluorometry of total cellular DNA in Acanthamoeba. J Histochem Cytochem. 1978 Sep;26(9):713–718. doi: 10.1177/26.9.361883. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J., van de Voorde H. Comparative study of six strains of Naegleria with special reference to nonpathogenic variants of Naegleria fowleri. J Protozool. 1977 May;24(2):304–309. doi: 10.1111/j.1550-7408.1977.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Duma R. J. Primary amoebic meningoencephalitis. CRC Crit Rev Clin Lab Sci. 1972 Jun;3(2):163–192. doi: 10.3109/10408367209151325. [DOI] [PubMed] [Google Scholar]
- Griffin J. L. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science. 1972 Nov 24;178(4063):869–870. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- Jager B. V., Stamm W. P. Brain abscesses caused by free-living amoeba probably of the genus Hartmannella in a patient with Hodgkin's disease. Lancet. 1972 Dec 23;2(7791):1343–1345. doi: 10.1016/s0140-6736(72)92781-x. [DOI] [PubMed] [Google Scholar]
- Leary J. F., Todd P. Laser cytophotometric detection of variations in spatial distribution of concanavalin-A cell surface receptors following viral infection. J Histochem Cytochem. 1977 Jul;25(7):908–912. doi: 10.1177/25.7.197163. [DOI] [PubMed] [Google Scholar]
- Martinez A. J., Markowitz S. M., Duma R. J. Experimental pneumonitis and encephalitis caused by acanthamoeba in mice: pathogenesis and ultrastructural features. J Infect Dis. 1975 Jun;131(6):692–699. doi: 10.1093/infdis/131.6.692. [DOI] [PubMed] [Google Scholar]
- Naginton J., Watson P. G., Playfair T. J., McGill J., Jones B. R., Steele A. D. Amoebic infection of the eye. Lancet. 1974 Dec 28;2(7896):1537–1540. doi: 10.1016/s0140-6736(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., Kilpatrick T., Willaert E., Capron A. Serologic analyses of cell-surface antigens of Acanthamoeba spp. with plasma membrane antisera. J Protozool. 1977 May;24(2):316–324. doi: 10.1111/j.1550-7408.1977.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., Tyndall R. L., Coutant C. C., Willaert E. Isolation of the etiological agent of primary amoebic meningoencephalitis from artifically heated waters. Appl Environ Microbiol. 1977 Dec;34(6):701–705. doi: 10.1128/aem.34.6.701-705.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara G. S., Balamuth W. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J Protozool. 1975 May;22(2):245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., COONS A. H. Fluorescent antibody studies with agents of varicella and herpes zoster propagated in vitro. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):789–794. doi: 10.3181/00379727-86-21235. [DOI] [PubMed] [Google Scholar]
- Wellings F. M., Amuso P. T., Chang S. L., Lewis A. L. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977 Dec;34(6):661–667. doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaert E. Isolement et culture in vitro des amibes du genre Naegleria. Ann Soc Belges Med Trop Parasitol Mycol. 1971;51(6):701–708. [PubMed] [Google Scholar]
- Willaert E., Jamieson A., Jadin J. B., Anderson K. Epidemiological and immunoelectrophoretic studies on human and environmental strains of Naegleria fowleri. Ann Soc Belg Med Trop. 1974;54(4-5):333–342. [PubMed] [Google Scholar]


