Abstract
Heterotrophic bacteria, yeasts, fungi, plants, and animal breath were investigated as possible sources of N2O. Microbes found to produce N2O from NO3− but not consume it were: (i) all of the nitrate-respiring bacteria examined, including strains of Escherichia, Serratia, Klebsiella, Enterobacter, Erwinia, and Bacillus; (ii) one of the assimilatory nitrate-reducing bacteria examined, Azotobacter vinelandii, but not Azotobacter macrocytogenes or Acinetobacter sp.; and (iii) some but not all of the assimilatory nitrate-reducing yeasts and fungi, including strains of Hansenula, Rhodotorula, Aspergillus, Alternaria, and Fusarium. The NO3−-reducing obligate anaerobe Clostridium KDHS2 did not produce N2O. Production of N2O occurred only in stationary phase. The nitrate-respiring bacteria produced much more N2O than the other organisms, with yields of N2O ranging from 3 to 36% of 3.5 mM NO3−. Production of N2O was apparently not regulated by ammonium and was not restricted to aerobic or anaerobic conditions. Plants do not appear to produce N2O, although N2O was found to arise from some damaged plant tops, probably due to microbial growth. Concentrations of N2O above the ambient level in the atmosphere were found in human breath and appeared to increase after a meal of high-nitrate food.
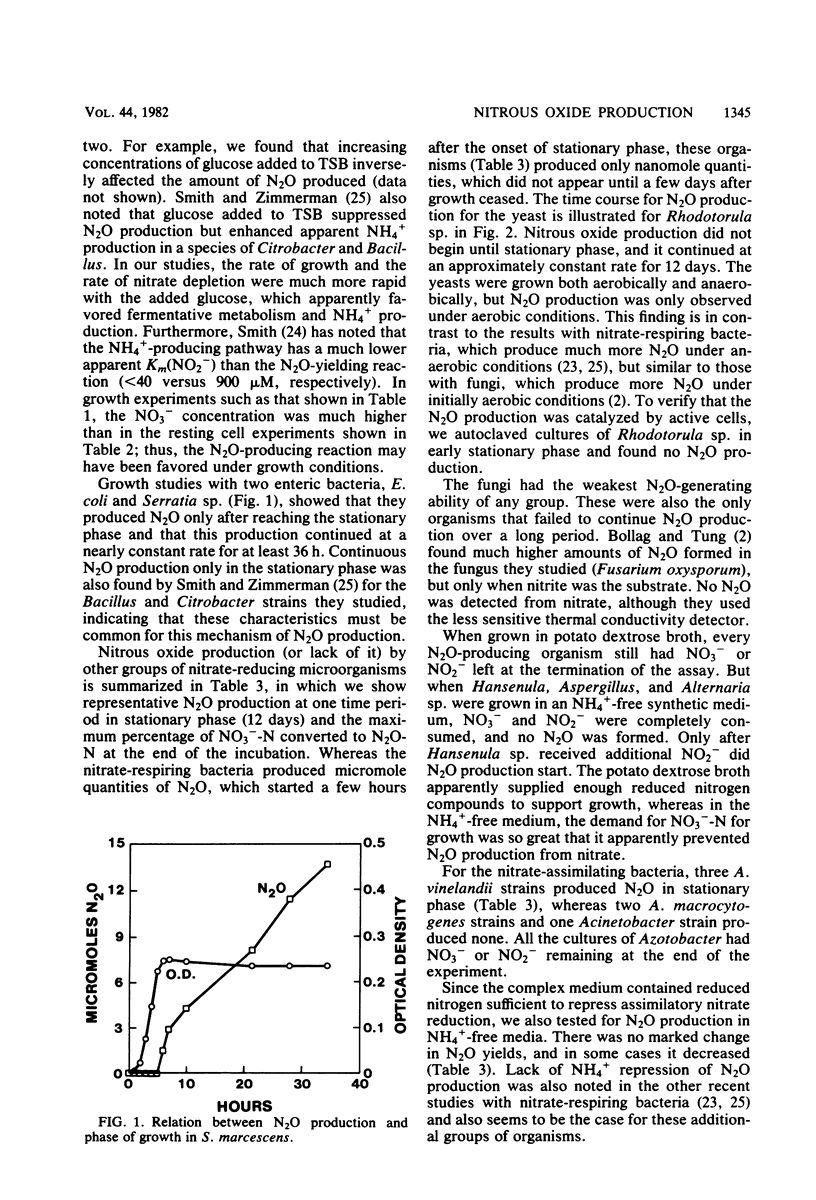
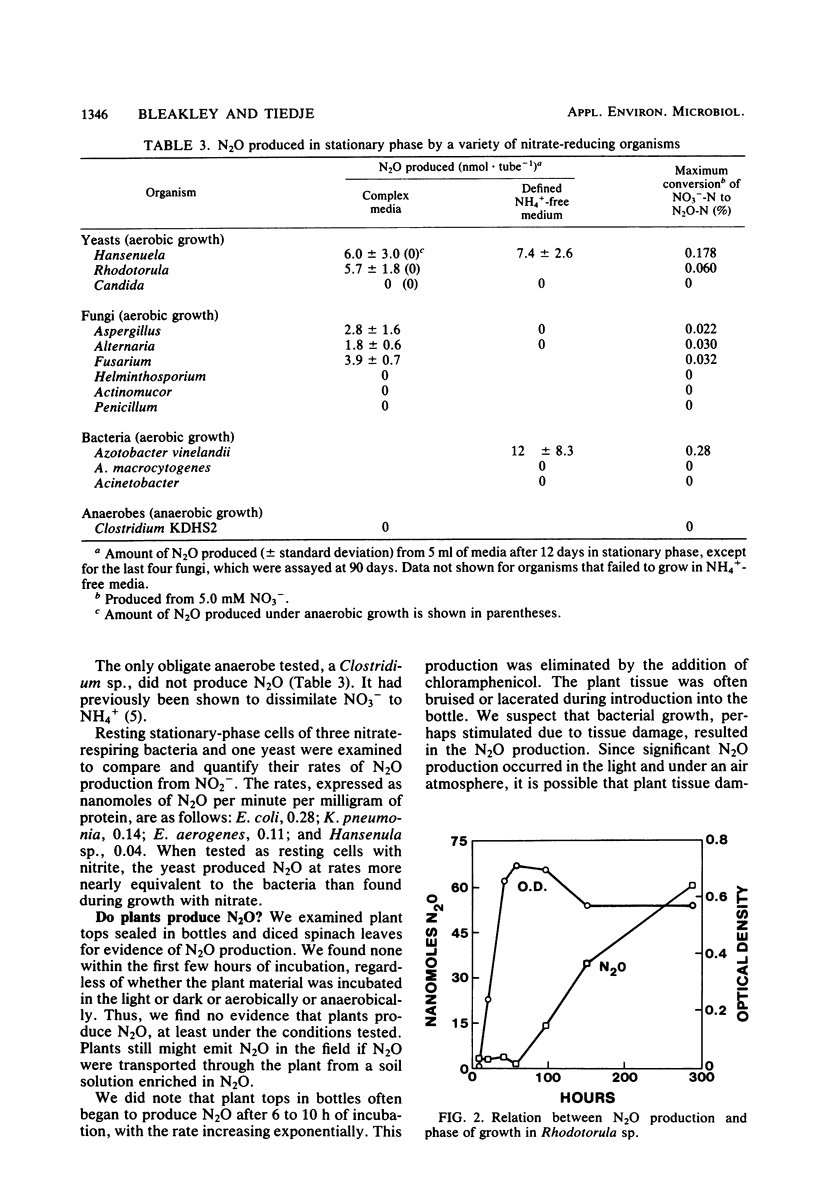
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackmer A. M., Bremner J. M., Schmidt E. L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980 Dec;40(6):1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E. Evolution of Nitrogen Oxide(s) during In Vivo Nitrate Reductase Assay of Soybean Leaves. Plant Physiol. 1981 Dec;68(6):1488–1493. doi: 10.1104/pp.68.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol. 1981 Mar;41(3):705–709. doi: 10.1128/aem.41.3.705-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Physiological factors affecting transformation of Azotobacter vinelandii. J Bacteriol. 1976 Mar;125(3):1080–1087. doi: 10.1128/jb.125.3.1080-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R., Todd R., Waide J. Microtechnique for most-probable-number analysis. Appl Environ Microbiol. 1977 Mar;33(3):675–680. doi: 10.1128/aem.33.3.675-680.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S. Dissimilatory Reduction of NO(2) to NH(4) and N(2)O by a Soil Citrobacter sp. Appl Environ Microbiol. 1982 Apr;43(4):854–860. doi: 10.1128/aem.43.4.854-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. C., Yung Y. L., Lacis A. A., Mo T., Hansen J. E. Greenhouse Effects due to Man-Mad Perturbations of Trace Gases. Science. 1976 Nov 12;194(4266):685–690. doi: 10.1126/science.194.4266.685. [DOI] [PubMed] [Google Scholar]


