Abstract
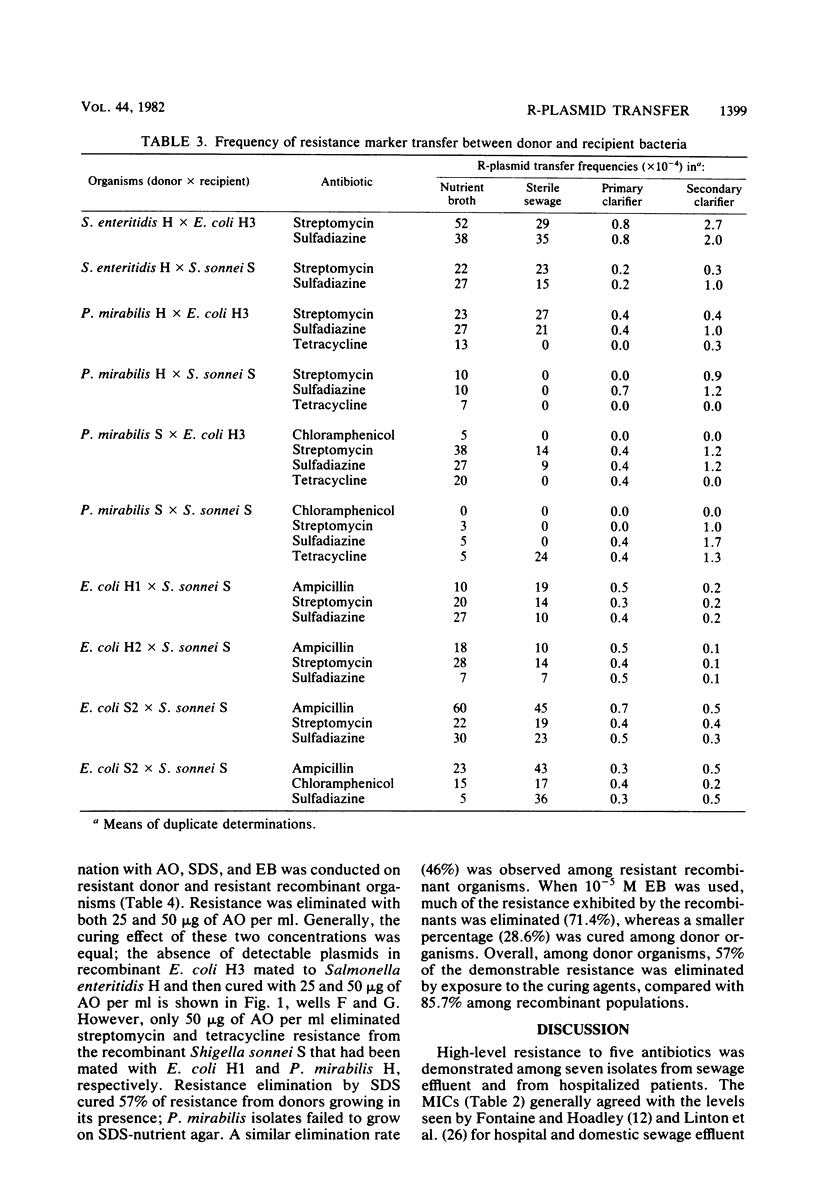
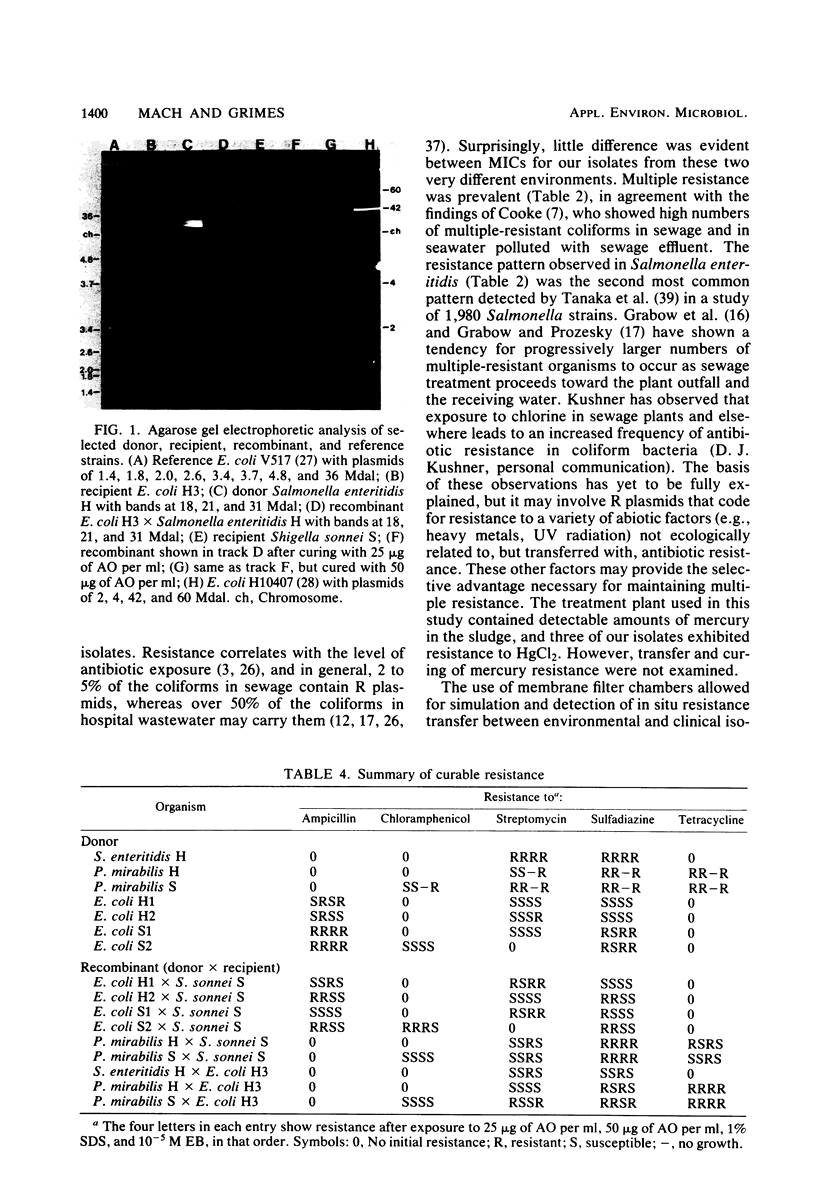
Enteric bacteria have been examined for their ability to transfer antibiotic resistance in a wastewater treatment plant. Resistant Salmonella enteritidis, Proteus mirabilis, and Escherichia coli were isolated from clinical specimens and primary sewage effluent. Resistance to ampicillin, chloramphenicol, streptomycin, sulfadiazine, and tetracycline was demonstrated by spread plate and tube dilution techniques. Plasmid mediation of resistance was shown by ethidium bromide curing, agarose gel electrophoresis, and direct cell transfer. Each donor was mated with susceptible E. coli and Shigella sonnei. Mating pairs (and recipient controls) were suspended in unchlorinated primary effluent that had been filtered and autoclaved. Suspensions were added to membrane diffusion chambers which were then placed in the primary and secondary setting tanks of the wastewater treatment plant. Resistant recombinants were detected by replica plating nutrient agar master plates onto xylose lysine desoxycholate agar plates that contained per milliliter of medium 10 micrograms of ampicillin, 30 micrograms of chloramphenicol, 10 micrograms of streptomycin, 100 micrograms of sulfadiazine, or 30 micrograms of tetracycline. Mean transfer frequencies for laboratory matings were 2.1 X 10(-3). In situ matings for primary and secondary settling resulted in frequencies of 4.9 X 10(-5) and 7.5 X 10(-5), respectively. These values suggest that a significant level of resistance transfer occurs in wastewater treatment plants in the absence of antibiotics as selective agents.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Nakano M., Inuzuka M., Tomoeda M. Specific role of sex pili in the effective eliminatory action of sodium dodecyl sulfate on sex and drug resistance factors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1114–1124. doi: 10.1128/jb.109.3.1114-1124.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Gillespie W. A., Richmond M. H. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):461–473. doi: 10.1099/00222615-6-4-461. [DOI] [PubMed] [Google Scholar]
- Armstrong J. L., Shigeno D. S., Calomiris J. J., Seidler R. J. Antibiotic-resistant bacteria in drinking water. Appl Environ Microbiol. 1981 Aug;42(2):277–283. doi: 10.1128/aem.42.2.277-283.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M. D. Antibiotic resistance among coliform and fecal coliform bacteria isolated from sewage, seawater, and marine shellfish. Antimicrob Agents Chemother. 1976 Jun;9(6):879–884. doi: 10.1128/aac.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullum J., Collins J. F., Broda P. The spread of plasmids in model populations of Escherichia coli K12. Plasmid. 1978 Sep;1(4):545–556. doi: 10.1016/0147-619x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Datta N. Drug resistance and R factors in the bowel bacteria of London patients before and after admission to hospital. Br Med J. 1969 May 17;2(5654):407–411. doi: 10.1136/bmj.2.5654.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Gorden R. W. Modification of membrane diffusion chambers for deep-water studies. Appl Environ Microbiol. 1977 Jan;33(1):207–210. doi: 10.1128/aem.33.1.207-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine T. D., 3rd, Hoadley A. W. Transferable drug resistance associated with coliforms isolated from hospital and domestic sewage. Health Lab Sci. 1976 Oct;13(4):238–245. [PubMed] [Google Scholar]
- Gardner P., Smith D. H. Studies on the epidemiology of resistance (R) factors. I. Analysis of Klebsiella isolates in a general hospital. II. A prospective study of R factor transfer in the host. Ann Intern Med. 1969 Jul;71(1):1–9. doi: 10.7326/0003-4819-71-1-1. [DOI] [PubMed] [Google Scholar]
- Grabow W. O., Prozesky O. W. Drug resistance of coliform bacteria in hospital and city sewage. Antimicrob Agents Chemother. 1973 Feb;3(2):175–180. doi: 10.1128/aac.3.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada H. S., Sizemore R. K. Incidence of Plasmids in Marine Vibrio spp. Isolated from an Oil Field in the Northwestern Gulf of Mexico. Appl Environ Microbiol. 1981 Jan;41(1):199–202. doi: 10.1128/aem.41.1.199-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel R. P., Miskell P. W., Altemeier W. A. Antibiotic resistance transfer from nonpathogenic to pathogenic bacteria. Surgery. 1977 Sep;82(3):382–385. [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Devaud M., Largiadér F., Binswanger V. Acquisition of multiple antibiotic resistance by Salmonella dublin from the gramnegative hospital flora, in a kidney allograft recipient. Zentralbl Bakteriol Orig A. 1978 Sep;241(3):308–318. [PubMed] [Google Scholar]
- Levin B. R., Stewart F. M. Probability of establishing chimeric plasmids in natural populations of bacteria. Science. 1977 Apr 8;196(4286):218–220. doi: 10.1126/science.847470. [DOI] [PubMed] [Google Scholar]
- Linton K. B., Richmond M. H., Bevan R., Gillespie W. A. Antibiotic resistance and R factors in coliform bacilli isolated from hospital and domestic sewage. J Med Microbiol. 1974 Feb;7(1):91–103. doi: 10.1099/00222615-7-1-91. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McNicol L. A., Aziz K. M., Huq I., Kaper J. B., Lockman H. A., Remmers E. F., Spira W. M., Voll M. J., Colwell R. R. Isolation of drug-resistant Aeromonas hydrophila from aquatic environments. Antimicrob Agents Chemother. 1980 Mar;17(3):477–483. doi: 10.1128/aac.17.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W. D., Miller R. V., Sayler G. S. Frequency of F116-mediated transduction of Pseudomonas aeruginosa in a freshwater environment. Appl Environ Microbiol. 1978 Nov;36(5):724–730. doi: 10.1128/aem.36.5.724-730.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury V., Hedges R. W., Datta N. Two modes of "curing" transmissible bacterial plasmids. J Gen Microbiol. 1972 May;70(3):443–452. doi: 10.1099/00221287-70-3-443. [DOI] [PubMed] [Google Scholar]
- Sato G., Furuta Y., Kodama H., Iwao T., Oka M. Enzootic occurrence of chloramphenicol-resistant Salmonella typhimurium var copenhagen in calf population. Am J Vet Res. 1975 Jun;36(6):839–841. [PubMed] [Google Scholar]
- Shaw D. R., Cabelli V. J. R-plasmid transfer frequencies from environmental isolates of Escherichia coli to laboratory and fecal strains. Appl Environ Microbiol. 1980 Oct;40(4):756–764. doi: 10.1128/aem.40.4.756-764.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton P., Anson A. E. Conjugal transfer of R-plasmid R1drd-19 in Escherichia coli below 22 degrees C. Appl Environ Microbiol. 1981 Nov;42(5):789–791. doi: 10.1128/aem.42.5.789-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore R. K., Colwell R. R. Plasmids carried by antibiotic-resistant marine bacteria. Antimicrob Agents Chemother. 1977 Sep;12(3):373–382. doi: 10.1128/aac.12.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. The effect on virulence of transferring R factors to Salmonella typhimurium in vivo. J Med Microbiol. 1972 Nov;5(4):451–458. doi: 10.1099/00222615-5-4-451. [DOI] [PubMed] [Google Scholar]
- Sturtevant A. B., Jr, Feary T. W. Incidence of infectious drug resistance among lactose-fermenting bacteria isolated from raw and treated sewage. Appl Microbiol. 1969 Nov;18(5):918–924. doi: 10.1128/am.18.5.918-924.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot H. W., Jr, Yamamoto D. K., Smith M. W., Seidler R. J. Antibiotic resistance and its transfer among clinical and nonclinical Klebsiella strains in botanical environments. Appl Environ Microbiol. 1980 Jan;39(1):97–104. doi: 10.1128/aem.39.1.97-104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Ikemura K., Tsunoda M., Sasagawa I., Mitsuhashi S. Drug resistance and distribution of R factors in Salmonella strains. Antimicrob Agents Chemother. 1976 Jan;9(1):61–64. doi: 10.1128/aac.9.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantanabe T., Aoki T., Ogata Y., Egusa S. Anbtibiotics and drug resistance in animals. R factors related to fish culturing. Ann N Y Acad Sci. 1971 Jun 11;182:383–410. doi: 10.1111/j.1749-6632.1971.tb30674.x. [DOI] [PubMed] [Google Scholar]



