Abstract
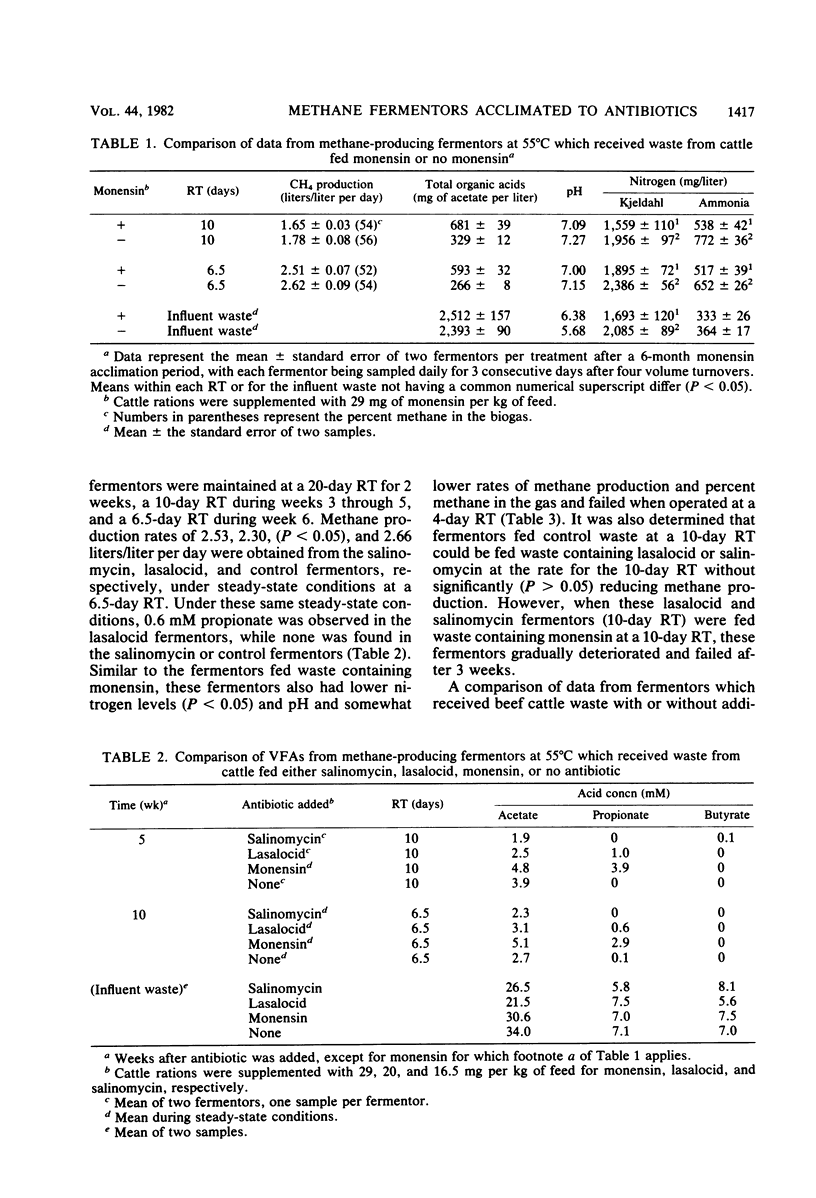
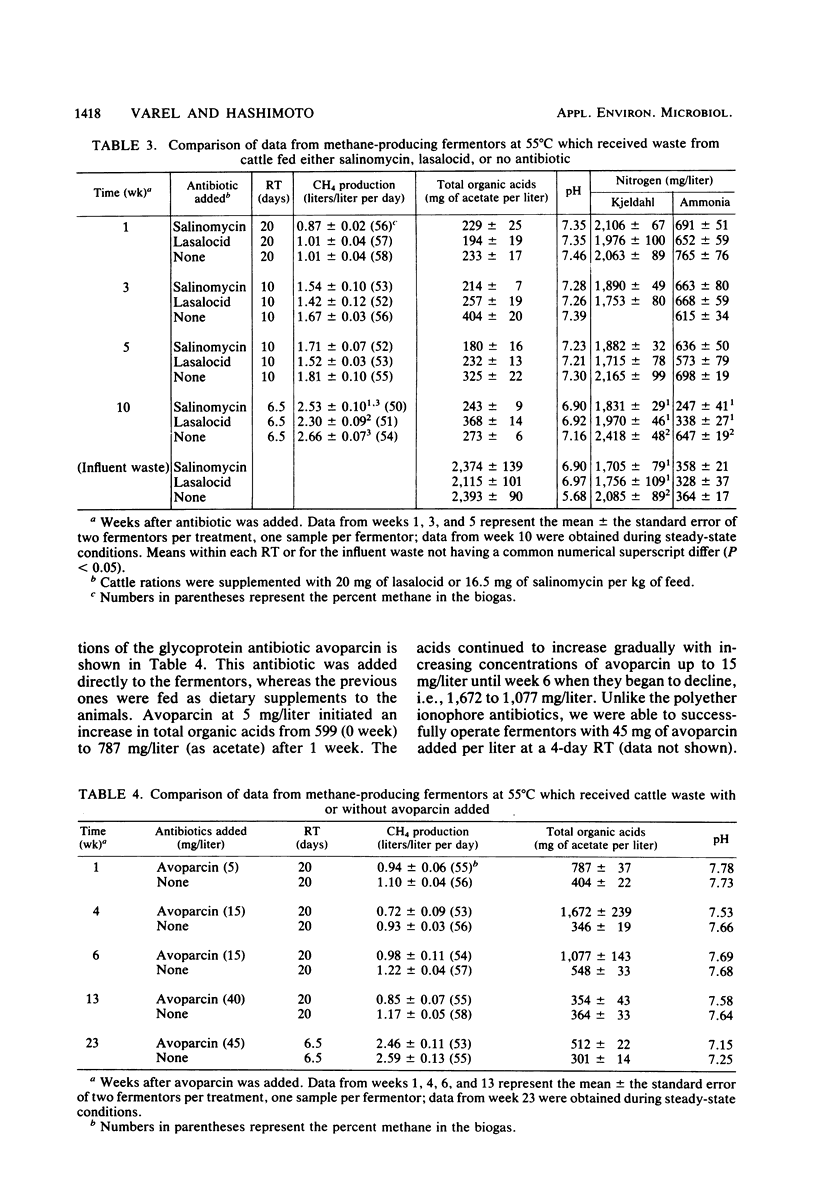
The ability of microorganisms to ferment waste from cattle fed monensin, lasalocid, or salinomycin to methane was determined. Continuously mixed anaerobic fermentors with 3-liter working volumes at 55°C were used; fermentors were fed once per day. Initially, all fermentors were fed waste without antibiotics at 6% volatile solids (VSs, organic matter) and a 20-day retention time (RT) for 60 days. Waste from animals fed monensin, lasalocid, or salinomycin at 29, 20, and 16.5 mg per kg of feed, respectively, was added to duplicate fermentors at the above VSs, and RT. Avoparcin (5 to 45 mg/liter) was not fed to animals but was added directly to duplicate fermentors. Lasalocid and salinomycin had minimal effects on the rate of methane production at RTs of 20 days and later at 6.5 days. Avoparcin caused an increase in organic acids from 599 to 1,672 mg/liter (as acetate) after 4 weeks, but by 6 weeks, acid concentrations declined and the rate of methane production was similar to controls at a 6.5-day RT. The monensin fermentors stopped producing methane 3 weeks after antibiotic addition. However, after a 6-month acclimation period, the microorganisms apparently adapted, and methane production rates of 1.65 and 2.51 liters per liter of fermentor volume per day were obtained with 6% VSs, and RTs of 10 and 6.5 days, respectively. This compares with 1.78 and 2.62 liters/liter per day for controls (P > 0.05). All fermentors that were fed waste containing antibiotics had lower pH values and ammonia and alkalinity concentrations, suggesting less buffering capacity and protein catabolism than in controls. Acclimation results obtained with fermentors at 35°C were similar to those for fermentors at 55°C. These studies indicate that waste from cattle fed these selected growth-promoting antibiotics can be thermophilically fermented to methane at RTs of 6.5 days or longer and VS concentrations of 6%, at rates comparable to waste without antibiotics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K. J., Zabransky R. J. Modification of the gas-liquid chromatography procedure and evaluation of a new column packing material for the identification of anaerobic bacteria. J Clin Microbiol. 1975 Jul;2(1):1–7. doi: 10.1128/jcm.2.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L. Monensin and dichloroacetamide influences on methane and volatile Fatty Acid production by rumen bacteria in vitro. Appl Environ Microbiol. 1979 Feb;37(2):283–288. doi: 10.1128/aem.37.2.283-288.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nevel C. J., Demeyer D. I. Effect of monensin on rumen metabolism in vitro. Appl Environ Microbiol. 1977 Sep;34(3):251–257. doi: 10.1128/aem.34.3.251-257.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Hashimoto A. G., Chen Y. R. Effect of temperature and retention time on methane production from beef cattle waste. Appl Environ Microbiol. 1980 Aug;40(2):217–222. doi: 10.1128/aem.40.2.217-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Hashimoto A. G. Effect of dietary monensin or chlortetracycline on methane production from cattle waste. Appl Environ Microbiol. 1981 Jan;41(1):29–34. doi: 10.1128/aem.41.1.29-34.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Isaacson H. R., Bryant M. P. Thermophilic methane production from cattle waste. Appl Environ Microbiol. 1977 Feb;33(2):298–307. doi: 10.1128/aem.33.2.298-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstone H. D., Davis C. L., Bryant M. P. Effect of monensin on breakdown of protein by ruminal microorganisms in vitro. J Anim Sci. 1981 Sep;53(3):803–809. doi: 10.2527/jas1981.533803x. [DOI] [PubMed] [Google Scholar]


