Abstract
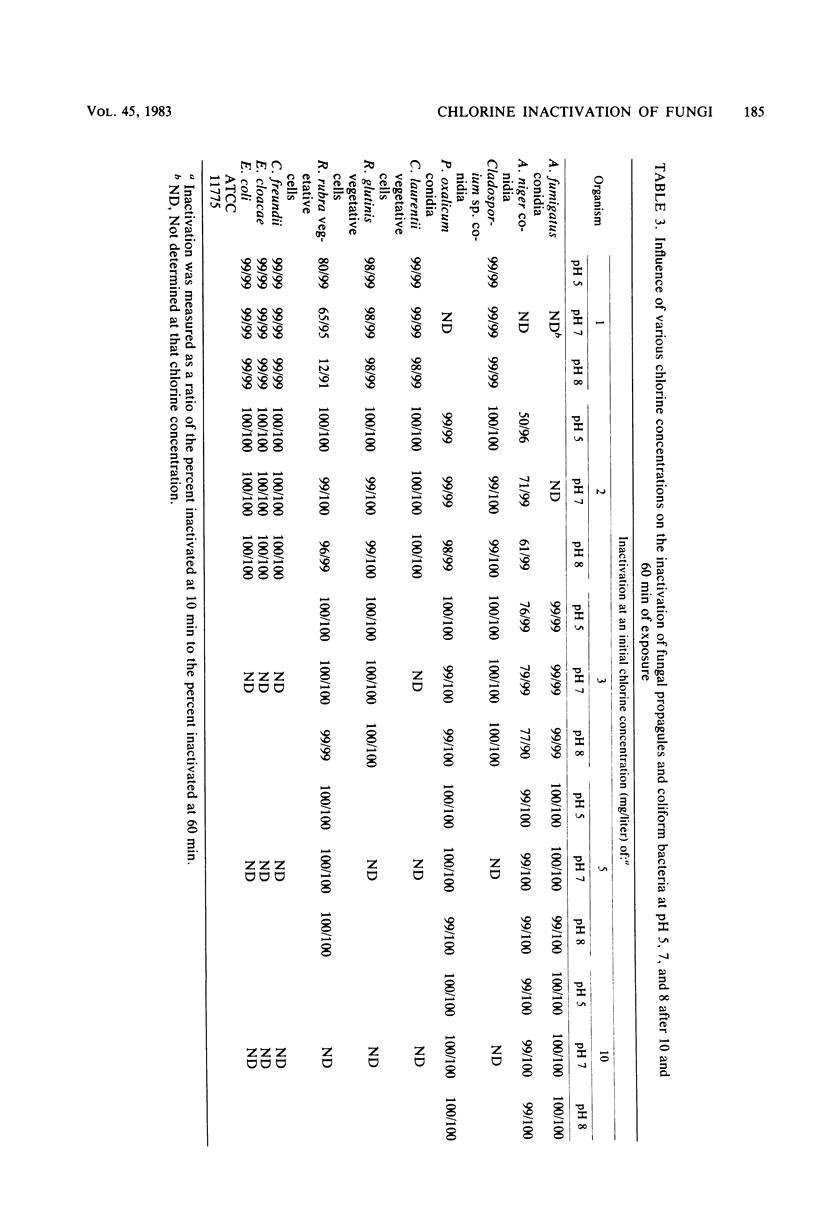
Conidia of filamentous fungi, vegetative yeast cells, and coliform bacteria were tested to determine their chlorine demand and their sensitivity to chlorine inactivation. Levels of chlorine demand for the various conidia, yeast, and coliforms were, respectively, 3.6 × 10−9 to 3.2 × 10−8, 1.2 × 10−9 to 8.0 × 10−9, and 2.5 × 10−11 to 6.3 × 10−10 mg of chlorine per propagule. Preliminary evidence suggests that the chlorine demand per propagule increases as the number of propagules per milliliter decreases. In general, conidia showed greatest resistance to chlorine inactiviation, followed by the yeast and coliforms. Inactivation by chlorine was influenced by pH, with inactivation (chlorine activity) falling in the order pH 5 > 7 > 8.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck J. D., Bubucis P. M. Membrane filter procedure for enumeration of Candida albicans in natural waters. Appl Environ Microbiol. 1978 Feb;35(2):237–242. doi: 10.1128/aem.35.2.237-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursons R. T., Brown T. J., Keys E. A. Effect of disinfectants on pathogenic free-living amoebae: in axenic conditions. Appl Environ Microbiol. 1980 Jul;40(1):62–66. doi: 10.1128/aem.40.1.62-66.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonckheere J., van de Voorde H. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl Environ Microbiol. 1976 Feb;31(2):294–297. doi: 10.1128/aem.31.2.294-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht R. S., Weber M. J., Salter B. L., Schmidt C. A. Comparative inactivation of viruses by chlorine. Appl Environ Microbiol. 1980 Aug;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C. N., Engelbrecht R. S. Physiological alterations of vegetative microorganisms resulting form chlorination. J Water Pollut Control Fed. 1980 Jul;52(7):1976–1989. [PubMed] [Google Scholar]
- Jarroll E. L., Bingham A. K., Meyer E. A. Effect of chlorine on Giardia lamblia cyst viability. Appl Environ Microbiol. 1981 Feb;41(2):483–487. doi: 10.1128/aem.41.2.483-487.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi R. M., Knuth S., Lundström K. Actinomycetes and fungi in surface waters and in potable water. Appl Environ Microbiol. 1982 Feb;43(2):378–388. doi: 10.1128/aem.43.2.378-388.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Dutka B. J. Comparison of various brands of membrane filters for their ability to recover fungi from water. Appl Environ Microbiol. 1976 Sep;32(3):445–447. doi: 10.1128/aem.32.3.445-447.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L. R., Waites W. M. The effect of chlorine on spores of Clostridium bifermentans, Bacillus subtilis and Bacillus cereus. J Gen Microbiol. 1975 Aug;89(2):337–344. doi: 10.1099/00221287-89-2-337. [DOI] [PubMed] [Google Scholar]


