Abstract
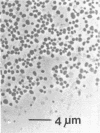
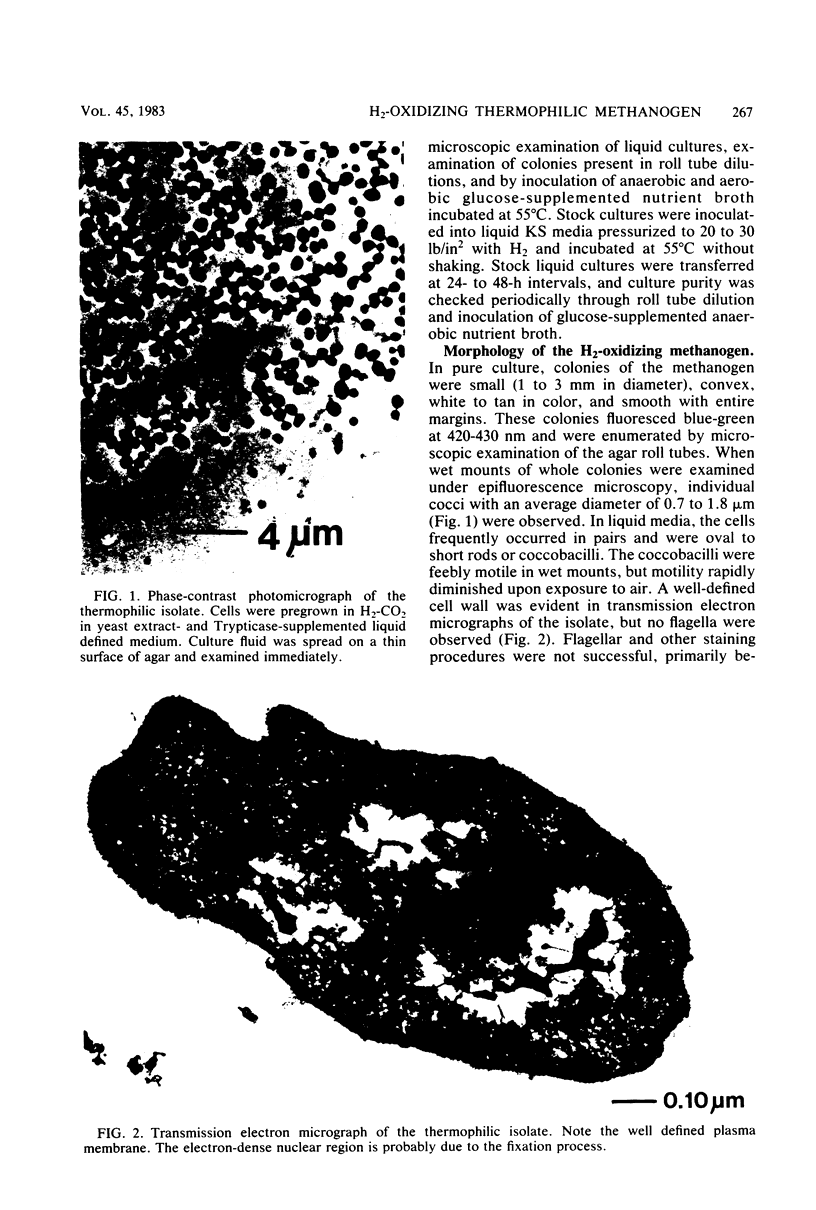
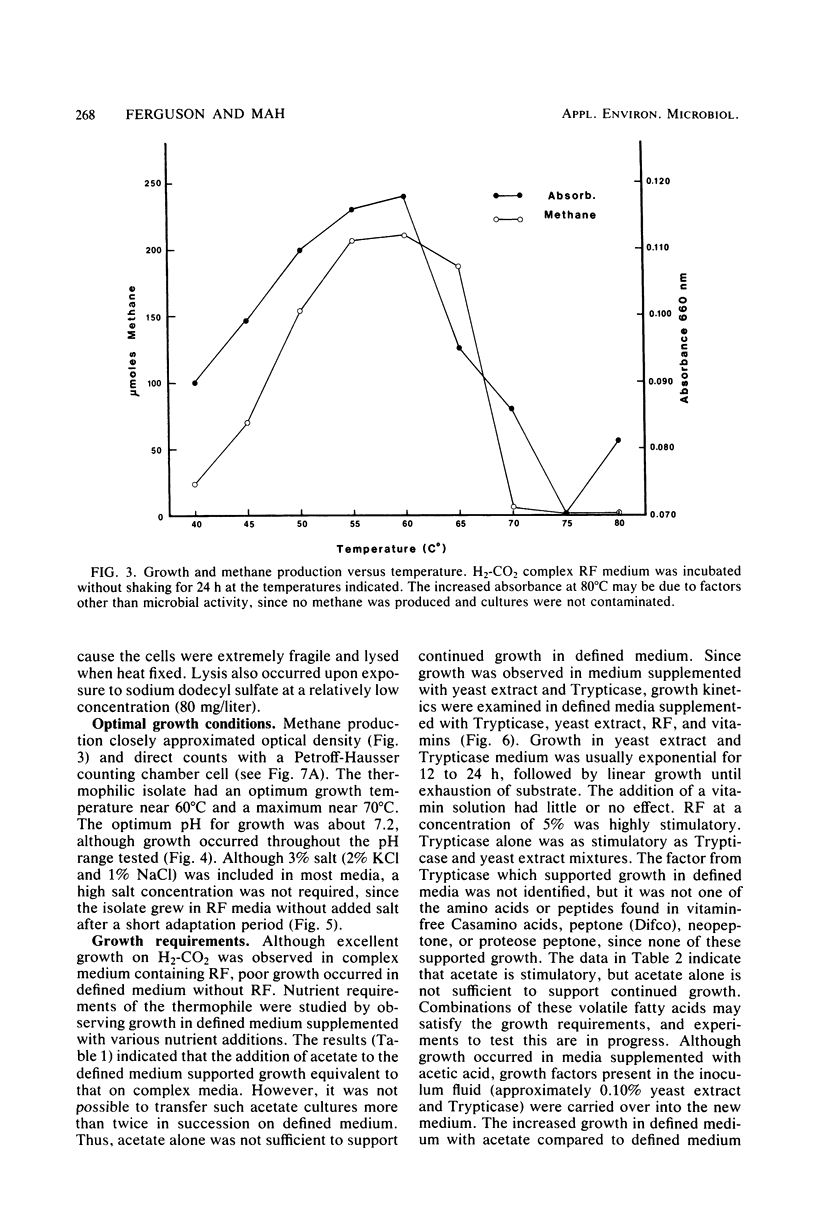
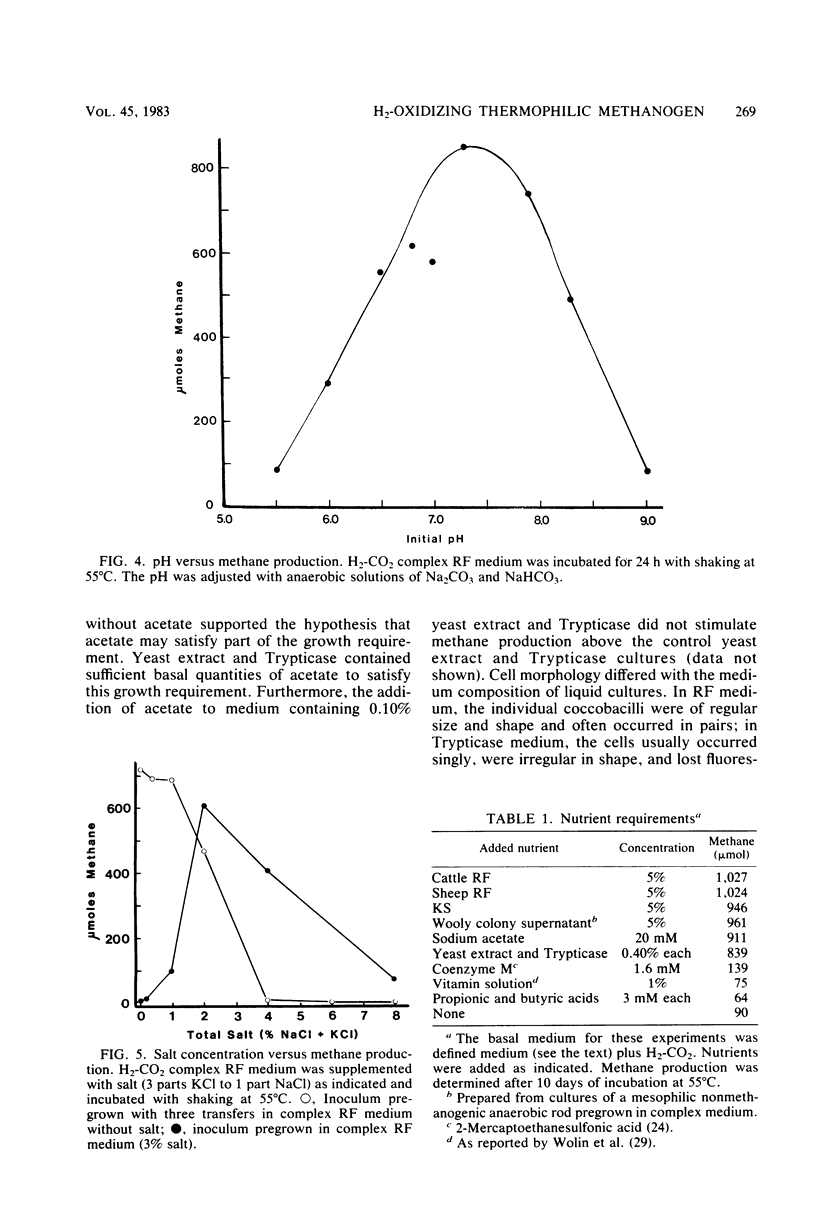
A thermophilic methanogen was isolated from enrichment cultures originally inoculated with sludge from an anaerobic kelp digester (55°C). This isolate exhibited a temperature optimum of 55 to 60°C and a maximum near 70°C. Growth occurred throughout the pH range of 5.5 to 9.0, with optimal growth near pH 7.2. Although 4% salt was present in the isolation medium, salt was not required for optimal growth. The thermophile utilized formate or H2-CO2 but not acetate, methanol, or methylamines for growth and methanogenesis. Growth in complex medium was very rapid, and a minimum doubling time of 1.8 h was recorded in media supplemented with rumen fluid. Growth in defined media required the addition of acetate and an unknown factor(s) from digester supernatant, rumen fluid, or Trypticase. Cells in liquid culture were oval to coccoid, 0.7 to 1.8 μm in diameter, often occurring in pairs. The cells were easily lysed upon exposure to oxygen or 0.08 mg of sodium dodecyl sulfate per ml. The isolate was sensitive to tetracycline and chloramphenicol but not penicillin G or cycloserine. The DNA base composition was 59.69 mol% guanine plus cytosine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baresi L., Mah R. A., Ward D. M., Kaplan I. R. Methanogenesis from acetate: enrichment studies. Appl Environ Microbiol. 1978 Jul;36(1):186–197. doi: 10.1128/aem.36.1.186-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway de Macario E., Wolin M. J., Macario A. J. Antibody analysis of relationships among methanogenic bacteria. J Bacteriol. 1982 Jan;149(1):316–319. doi: 10.1128/jb.149.1.316-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Bowers B., Stadtman T. C. Methanococcus vannielii: ultrastructure and sensitivity to detergents and antibiotics. J Bacteriol. 1977 Jun;130(3):1357–1363. doi: 10.1128/jb.130.3.1357-1363.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty D. G., Bianchi A. J. Isolement de deux souches méthanogènes thermophiles appartenant au genre Methanobacterium. C R Seances Acad Sci III. 1981 Jan 5;292(1):41–43. [PubMed] [Google Scholar]
- Paynter M. J., Hungate R. E. Characterization of Methanobacterium mobilis, sp. n., isolated from the bovine rumen. J Bacteriol. 1968 May;95(5):1943–1951. doi: 10.1128/jb.95.5.1943-1951.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Fuson G. B., Phaff H. J. Genome comparison in yeast systematics: delimitation of species within the genera Schwanniomyces, Saccharomyces, Debaryomyces, and Pichia. Microbiol Rev. 1978 Mar;42(1):161–193. doi: 10.1128/mr.42.1.161-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnow P. H., Gunnarsson L. A. Sulfide-dependent methane production and growth of a thermophilic methanogenic bacterium. Appl Environ Microbiol. 1981 Oct;42(4):580–584. doi: 10.1128/aem.42.4.580-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. H., HUNGATE R. E. Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol. 1958 Jun;75(6):713–718. doi: 10.1128/jb.75.6.713-718.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. X. A new formate-decomposing bacterium, Methanococcus vannielii. J Bacteriol. 1951 Sep;62(3):269–280. doi: 10.1128/jb.62.3.269-280.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbeck K. A., Ward D. M. Fate of immediate methane precursors in low-sulfate, hot-spring algal-bacterial mats. Appl Environ Microbiol. 1981 Mar;41(3):775–782. doi: 10.1128/aem.41.3.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Metabolism of formate in Methanobacterium formicicum. J Bacteriol. 1980 Jun;142(3):800–807. doi: 10.1128/jb.142.3.800-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., McBride B. C., Wolfe R. S., Bryant M. P. Coenzyme M, essential for growth of a rumen strain of Methanobacterium ruminantium. J Bacteriol. 1974 Nov;120(2):974–975. doi: 10.1128/jb.120.2.974-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. T., Pirt S. J. Nutrition and factors limiting the growth of a methanogenic bacterium (Methanobacterium thermoautotrophicum). Arch Microbiol. 1977 May 13;113(1-2):17–22. doi: 10.1007/BF00428574. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Hashimoto A. G., Chen Y. R. Effect of temperature and retention time on methane production from beef cattle waste. Appl Environ Microbiol. 1980 Aug;40(2):217–222. doi: 10.1128/aem.40.2.217-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Isaacson H. R., Bryant M. P. Thermophilic methane production from cattle waste. Appl Environ Microbiol. 1977 Feb;33(2):298–307. doi: 10.1128/aem.33.2.298-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Henning D. L. Methanobacterium arbophilicum sp.nov. An obligate anaerobe isolated from wetwood of living trees. Antonie Van Leeuwenhoek. 1975;41(4):543–552. doi: 10.1007/BF02565096. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]