Abstract
Erythropoietin (Epo) is essential for formation of mature red blood cells (RBC). However, the function of Epo receptor (EpoR)-dependent signaling pathways in the regulation of erythropoiesis remains unclear. To determine whether specific Stat signals are required for RBC development, we changed the Stat signaling specificity of the EpoR. The wild-type EpoR activates only Stat5. Thus, we substituted the major Stat5 binding sites (residues 343 and 401) in the EpoR cytoplasmic region with the Stat3 binding/activation motif from gp130. We demonstrated that activated EpoRs containing a single substitution stimulate Stat5 and Stat3, whereas an EpoR with both substitutions stimulates Stat3 but not Stat5. We then determined the ability of these receptors to support fetal liver and adult erythropoiesis. Our results show that erythropoiesis is stimulated by EpoRs that activate Stat5, both Stat5 and Stat3, or Stat3 in place of Stat5. These findings demonstrate that the specificity of EpoR Stat signaling is not essential for RBC development.
INTRODUCTION
Red blood cell (RBC) development in the bone marrow is critically dependent on the levels of circulating erythropoietin (Epo). This is dramatically demonstrated in humans with chronic renal failure, who lack normal Epo production in the kidney and exhibit severe anemia unless treated with recombinant Epo. Mice containing targeted disruptions of the Epo or Epo receptor (EpoR) gene (Epo−/− or EpoR−/− mice, respectively) also demonstrate severe defects in RBC production.(1–3) These animals die of anemia at approximately day 14 of gestation, a time that corresponds to the onset of definitive (adult) hematopoiesis in the fetal liver. Therefore, Epo and the EpoR are essential for definitive erythropoiesis in the fetal liver and adult bone marrow.
Several studies have suggested that Epo is not critical for commitment to the erythroid lineage.(4) The Epo−/−and EpoR−/− mice support these findings. Definitive erythroid progenitor cells are present in these animals, but they are arrested at the colony-forming unit–erythroid (CFU-E) stage of development and do not differentiate into mature erythrocytes.(1–3) The EpoR−/− mice do not contain any obvious defects in the development of other blood cell lineages, indicating that Epo is a lineage-specific growth factor.
The EpoR belongs to the cytokine receptor superfamily, sharing structural similarity in both extracellular and intracellular regions.(5,6) Like other members of the family, the EpoR stimulates multiple intracellular signaling proteins, including Jak2, SHP-1, and phosphoinositol 3-kinase.(7–9) The EpoR also specifically activates the Stat5a and Stat5b transcription factor proteins.(10,11) As Stat proteins are critical mediators of cytokine-specificsignals,(12,13) it was anticipated that Stat5 would be essential for erythropoiesis. Surprisingly, however, mice lacking Stat5a and Stat5b expression do not exhibit signs of anemia, and bone marrow cells derived from these animals give rise to normal levels of Epo-dependent erythroid colonies in ex vivo cultures.(14) These data demonstrate that Stat5a and Stat5b are not essential for erythropoiesis in the adult. However, the Stat5 proteins are required for efficient erythropoiesis in the developing embryo, as evidenced by the severe anemia found in day 13.5 embryos lacking Stat5a and Stat5b expression.(15) Therefore, erythroid progenitor cells appear to have developmentally distinct requirements for Stat5 proteins, which may reflect different proliferative demands at each stage of development.
Interestingly, signaling through other cytokine receptors can also support erythropoiesis. This has been demonstrated by studies of chimeric receptors, in which the extracellular region of the EpoR was fused to intracellular regions derived from other cytokine receptors (e.g., granulocyte colony-stimulating factor receptor [G-CSFR], growth hormone receptor [GHR], prolactin receptor, thrombopoietin receptor).(16,17) In addition, other cytokines can support the development of some erythroid colonies from EpoR−/− fetal liver cells in vitro, although the number and size of the colonies are reduced relative to wild-type cells cultured in Epo.(2,3) Collectively, these results show that signals from other cytokine receptors can compensate, in part, for EpoR-mediated signals. However, the cytokines and receptors used in these studies activate many of the same signaling pathways as the EpoR, including Stat5. Therefore, the precise function of specific signaling pathways in erythroid development remains unclear.
To examine the requirements for specific Stat signals during erythropoiesis, we generated EpoRs that activate Stat5, both Stat5 and Stat3, or Stat3 in place of Stat5. The ability of these receptors to support fetal liver and adult erythropoiesis was then determined. We found that erythropoiesis at both developmental stages was supported by EpoRs with altered Stat signaling. These results demonstrate that although the EpoR naturally activates only Stat5, this specificity is not required for RBC development.
MATERIALS AND METHODS
Generation of EpoR mutants and retroviral vectors
The murine EpoR was mutagenized as described(18) to substitute the Stat3 binding/activation motif from gp130(19) for the major Stat5 binding sites in the EpoR at residues 343 and 401.(10,11) This generated ER343-S3, ER401-S3, and ER343/401-S3, which contain single substitutions at position 343 or 401 or substitutions at both positions, respectively (Fig. 1). The cytoplasmic regions of ER343-S3, ER401-S3, and ER343/401-S3 were fused to the extracellular region of the constitutively active EpoR mutant R129C(20) to generate R129C/343-S3, R129C/401-S3, and R129C/343/401-S3. R129C-containing cDNAs were subcloned into the pMEX vector for expression studies in 32D cells or into the erythroleukemic spleen focus-forming virus (SFFV) for erythroid progenitor and animal studies.(21) Retroviruses were generated from SFFV-containing plasmids and were titered by quantitative anti-EpoR immunoblot analysis as previously described.(21,22)
FIG. 1.
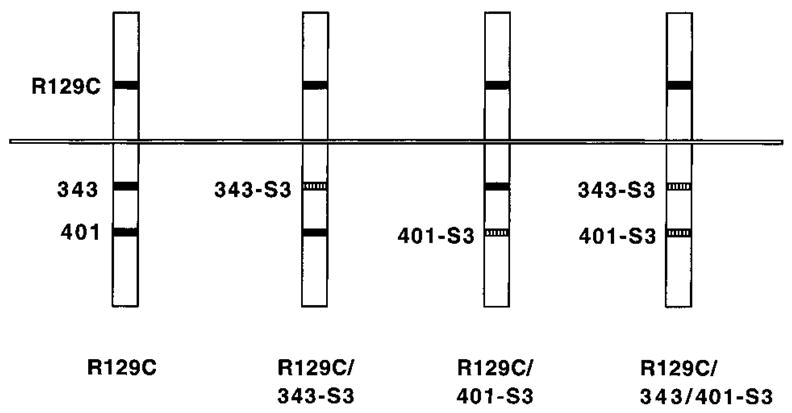
Diagram of R129C isoforms containing altered Stat binding regions. R129C is shown at left, with the R129C substitution and tyrosine residues (343 and 401) required for Stat5 activation indicated by black boxes. Other tyrosine residues in the receptor cytoplasmic region are not shown. R129C/343-S3 has the sequence GYMPQ (Stat3 binding site) substituted for residues 342–346, R129C/401-S3 has GYMPQ substituted for residues 400–404, and R129C/343/401-S3 has both substitutions (as indicated by hatched boxes). The substitutions preserve Y343 and Y401 but change residues at the −1, +1, +2, and +3 positions. All other residues are identical to wild-type (WT) EpoR.
Cell lines, culture conditions, and transfections
Interleukin-3 (IL-3)-dependent 32D cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 5% conditioned medium (RPMI/FBS/WEHI) from the WEHI 3B cell line; the latter was used as a source of IL-3. 32D cells were electroporated as described previously,(23) and transfected cells were selected by growth in RPMI/FBS/WEHI containing 750 μg/ml G418. Expression of the mutant EpoR was verified by EpoR immunoprecipitations from metabolically labeled cells or by immunoprecipitation and immunoblotting assays (not shown).
Epo stimulations, immunoprecipitations, and antiphosphotyrosine immunoblotting assays
Antisera specific for Stat5 or Stat3 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antiphosphotyrosine antibody 4G10 was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). To perform Epo stimulations, cells were cultured in serum-free RPMI for 4 h at 37°C, then stimulated with 20 U/ml for 8 min at 37°C. Stat proteins were immunoprecipitated from detergent cell extracts, and antiphosphotyrosine immunoblotting assays were performed as described previously.(24)
Ex vivo colony-forming assays, in vivo erythroid development assays, and RT-PCR analysis
Colony-forming assays were performed as described previously.(25) Briefly, pregnant DBA-2 mice (Charles River Laboratories, Bar Harbor, ME) were dissected at day 13 of gestation, and single cell suspensions were prepared from fetal livers. Cells (106) were washed three times in α medium, resuspended in medium containing fresh virus and 4 μg/ml Polybrene, and incubated for 37°C for 3 h. After infection, the cells were washed in α medium and replated in α medium containing 30% FBS (Sterile Systems, Logan, UT), 1% crystallized bovine serum albumin (BSA), 1.2% 1500 centipoise methylcellulose (1 poise = 0.1 Pa.sec) (Shinetsu Chemical, Tokyo, Japan), and 50 μM β-mercaptoethanol(Sigma, St. Louis, MO) at a cell concentration of 1 × 104/ml. CFU-E colonies were scored visually after 2 days.
For in vivo studies, adult mice were inoculated intravenously (i.v.) with a mixture of recombinant SFFV virus (SFF.R129C, SFF.R129C/343-S3, SFF.R129C/401-S3, or SFF.R129C/343/401-S3) and replication-competent Rauscher murine leukemia virus (MuLV) (at a 7:3 ratio), as described.(21) Erythropoiesis was monitored by weekly hematocrit determinations. Animals were sacrificed when their hematocrits reached 70–80, or at 8 weeks after infection, and terminal spleen weights were determined. Total RNA was isolated from spleens as previously described.(21) Complementary cDNA was synthesized with the Superscript kit (Life Technologies Gaithersburg, MD), according to the manufacturer’s instructions. PCR amplification was performed as previously described(21) with a sense primer corresponding to the Stat3 substitution at position 343 (5′-GGGTACATGCCTCAGGATAAG-3′) and an antisense primer from the SFFV vector (5′-CTGGAGGAGGAGGCTGAAGAG-3′) or with a sense primer from the Stat3 substitution at position 401 (5′-GGGTACATGCCTCAGGAC-3′) and the SFFV primer (see legend for Fig. 3).
FIG. 3.
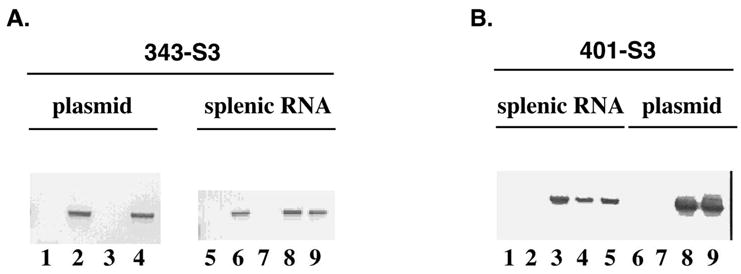
Detection of retrovirally transduced EpoR sequences in spleens from infected animals. (A) Plasmid DNA (lanes 1–4) or splenic RNA (lanes 5–9) were amplified by PCR or RT-PCR with a sense primer from the Stat3 site containing Y343 and an antisense primer from the SFFV vector. The lanes correspond to plasmids pSFF-R129C (lane 1) pSFF-R129C/343-S3 (lane 2), pSFF-R129C/401-S3 (lane 3), pSFF-R129C/343/401-S3 (lane 4), splenic RNA from an uninfected mouse (lane 5) or mice infected with SFF-R129C/343-S3 (lane 6), pSFF-R129C/401-S3 (lane 7), or pSFF-R129C/343/401-S3 (lanes 8 and 9). (B) A similar analysis was performed with a sense primer from the Stat3 site containing Y401 and the antisense primer from the SFFV vector. Results from the analysis of splenic RNA samples are shown in lanes 1–5, in the order indicated in A. Results from plasmid DNA analysis are shown in lanes 6–9, in the order indicated in A.
RESULTS
Signal transduction from EpoRs with altered Stat specificity
A schematic diagram of the EpoR mutants used in this study is shown in Figure 1. To confirm that the mutations confer the anticipated changes in Stat protein signaling specificity, we generated 32D cell lines expressing these EpoRs and analyzed Stat protein activation by immunoprecipitation and antiphosphotyrosine immunoblotting assays. The results demonstrate that R129C activates only Stat5, as expected, whereas R129C/343-S3 and R129C/401-S3 activate both Stat5 and Stat3 (Fig. 2). In contrast, R129C/343/401-S3, which contains substitutions at both Stat5 binding sites, activates only Stat3 (Fig. 2). Therefore, substitution of the EpoR Stat5 activation sites with Stat3 activation motifs alters Stat-dependent signal transduction from the receptor.
FIG. 2.
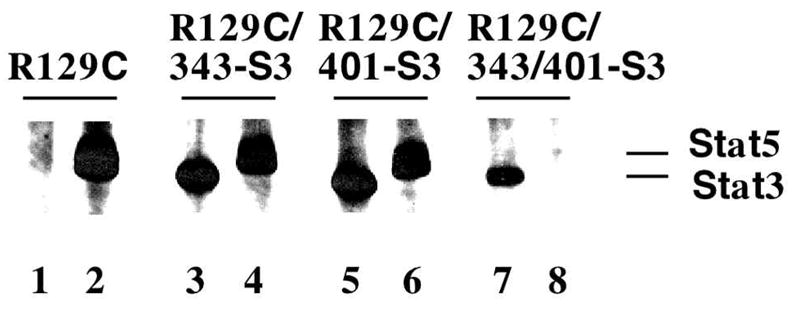
Stat signaling specificity of chimeric EpoR. 32D cells expressing R129C, R129C/343-S3, R129C/401-S3, or R129C/343/401-S3 were cultured in RPMI for 4 h, then stimulated with 20 U/ml Epo for 8 min at 37°C. Stat3 (lanes 1, 3, 5, 7) and Stat5 (lanes 2, 4, 6, 8) were immunoprecipitated from detergent cell extracts, and proteins were analyzed by antiphosphotyrosine immunoblots. A digital image of the autora-diogram is shown.
Fetal liver CFU-E development is supported by EpoRs that activate Stat3
Mice transduced with R129C-containing retrovirus develop massive erythrocytosis and splenomegaly.(21) R129C also supports Epo-independent erythropoiesis in ex vivo cultures.(25) Collectively, these results demonstrate that R129C signaling is dominant to the endogenous WT EpoR. Therefore, use of R129C-containing EpoR mutants is a powerful method to analyze EpoR signaling requirements in a wild-type genetic background.
To determine the function of EpoR-mediated Stat signals in fetal liver RBC development, we performed ex vivo colony-forming assays. Erythroid progenitor cells were isolated from fetal livers at day 13 of gestation and were infected with SFFV stocks carrying R129C, R129C/343-S3, R129C/401-S3, or R129C/343-S3/401-S3. Infected cells were plated in methylcellulose-containing cultures in the absence of Epo or other cytokines to ensure that the endogenously expressed WT EpoR or other cytokine receptors do not contribute to erythropoiesis. Because the titers of SFF-R129C/ER343-S3, SFF-R129C/ER401-S3, and SFF-R129C/ER343/401-S3 were reproducibly one-third to one-fourth that of SFF-R129C (data not shown), we also performed a set of infections with SFF-R129C that had been diluted 1:3 immediately prior to infection. As expected, R129C supportedEpo-independentCFU-E development(Table 1). Epo-independent CFU-E development was also stimulated by EpoRs containing one or more Stat3 binding sites. The differences observed in the efficiency of CFU-E formation between recombinant retroviruses are most likely due to differences in viral titer (Table 1; data not shown). Therefore, these data demonstrate that fetal liver CFU-E development is stimulated by EpoRs that activate Stat5, both Stat5 and Stat3, or Stat3 in place of Stat5.
Table 1.
Function of EpoRs with Alerted Stat Signaling in Fetal Erythropoiesisa
| Virus (dilution) | Epo-independent CFU-E |
|---|---|
| None | 5 ± 3 (0) |
| SFF-R129C | 82 ± 11 (77) |
| SFF-R129C (1:3) | 20 ± 4 (15) |
| SFF-R129C/343-S3 | 28 ± 1 (23) |
| SFF-R129C/401-S3 | 40 ± 7 (35) |
| SFF-R129C/343/401-S3 | 39 ± 7 (34) |
Fetal liver cells from day 13 mouse embryos were infected with retroviruses carrying constitutively active EpoR (as indicated). To adjust for differences in viral titer, a 1:3 dilution of SFF-R129C was also used. Erythroid colony-forming assays were performed as described in Materials and Methods. Mean colony numbers from triplicate cultures are shown for a representative experiment.
Adult erythropoiesis is stimulated by EpoRs with altered patterns of Stat activation
To examine the effect of altered EpoR Stat specificity on RBC development in the adult animal, 6–8-week-old mice were infected with SFFV stocks carrying R129C, R129C/343-S3, R129C/401-S3, or R129C/343-S3/401-S3. Erythropoiesis was monitored by weekly hematocrit determinations. In 75% of the animals infected with R129C/343-S3 or R129C/401-S3, hema tocrits of 70–80 were reached by 6–8 weeks after infection. Similarly, 75% of the animals infected with R129C/343/401-S3 had elevated hematocrit levels after 8 weeks, although the levels were lower than in animals infected with R129C, R129C/343-S3, or R129C/401-S3 (50–60 compared to 70–80) (Table 2). Spleen weight, an indicator of in vivo expansion of erythroid progenitor populations,(22) was also increased in 75% of the infected animals (Table 2).
Table 2.
Function of EpoRs with Altered Stat Signaling in Adult Erythropoiesisa
| Virus | Hematocrit (week) | Spleen weight (g) |
|---|---|---|
| None | 44 (6) | 0.15 |
| SFF-R129C | 75 (6) | 2.0 |
| 82 (6) | 2.2 | |
| SFF-R129C/343-S3 | 82 (8) | 0.9 |
| 75 (8) | 1.44 | |
| 72 (8) | d.b.a. | |
| No disease | — | |
| SFF-R129C/401-S3 | 73 (8) | 2.2 |
| 74 (8) | 3.4 | |
| 72 (8) | d.b.a. | |
| No disease | — | |
| SFF-R129C/343/401-S3 | 61 (8) | 2.2 |
| 55 (8) | 1.0 | |
| 59 (8) | 0.8 | |
| No disease | — |
Adult mice were infected with recombinant retroviruses as described in Materials and Methods. Blood samples were taken at weekly intervals after infection, and the packed RBC volumes (hematocrits) were measured. Hematocrits for individual animals are shown, with the time of determination (in weeks) indicated. Animals were killed once their hematocrit reached levels of 70–80, or at 8 weeks after infection. Spleens were removed and weighed to determine the extent of splenomegaly. Spleen weights for individual animals are indicated (in grams). Some animals died before analysis of the spleen (d.b.a.).
To confirm expression of the predicted EpoR isoforms in the diseased mice, RT-PCR analysis of splenic RNA isolated from diseased animals was performed using primers specific for the mutant Stat3 sites and SFFV sequences. In all cases examined, transcripts corresponding to only the transduced R129C isoform were detected (Fig. 3). Therefore, these results demonstrate that adult erythropoiesis is stimulated by EpoRs that activate Stat3.
DISCUSSION
We examined the requirement for EpoR-regulated Stat signals in fetal liver erythroid progenitors and during adult erythropoiesis in vivo. We found that EpoRs that activate both Stat5 and Stat3 or that activate Stat3 in place of Stat5 support the development of fetal liver erythroid progenitors and adult RBC. Therefore, although the wild-type EpoR signals through Stat5, this specificity is not essential for definitive erythropoiesis.
We altered EpoR-mediated Stat signal transduction by replacing the sequences surrounding EpoR tyrosines 343 and 401, the major sites of Stat5 binding and activation(10,11) with the Stat3 binding/activation site from gp130.(19) Both Stat5 and Stat3 were stimulated by EpoRs containing a single substitution, whereas Stat3 was activated in place of Stat5 in an EpoR containing both substitutions. Therefore, the Stat binding site substitutions selectively altered EpoR-dependent Stat signals. As cell type-specific factors can contribute to the specificity of cytokine receptor signal transduction, we analyzed the function of these EpoRs in primary erythroid cells. To accomplish this, we fused the cytoplasmic regions of EpoRs with altered Stat specificity to the extracellular region of the constitutively active EpoR, R129C, to generate dominantly acting receptor isoforms. This approach has been used previously to study EpoR functions, as it enables rapid analysis of receptor mutants in primary erythroid cells.(16,21,22,25,26)
ER343-S3, ER401-S3, and ER343/401-S3 support CFU-E development from fetal liver erythroid progenitors. Furthermore, mice infected with recombinant retroviruses develop erythrocytosis and splenomegaly, demonstrating that these receptor isoforms support erythropoiesis in the adult animal. Although the onset of disease was slower in animals infected with viruses carrying EpoRs with altered Stat specificity, this is likely to represent differences in viral titer, as these mutants consistently yielded lower titer virus than R129C. In support of this, dilution of the R129C retrovirus stock supported levels of CFU-E development that were equivalentto ER343-S3, ER401-S3, and ER343/401-S3. Therefore, the loss of Stat5 signaling and the gain of Stat3 signaling by the EpoR does not enhance or abrogate the development of fetal liver and adult erythroid progenitors.
The erythroid development we observed in ex vivo cultures is dependent on signals from the constitutively active mutant EpoRs and not on signals from the endogenous EpoR or other cytokine receptors, as these cultures were maintained in the absence of Epo or any other cytokine. This result is significant in light of the fact that erythroid cells appear to be less dependent on specific cytokine signals for their maturation than cells of other hematopoietic lineages. Progenitor cells isolated from EpoR−/−mice can be induced to undergo erythroid differentiation in the presence of other cytokines.(2,3) In addition, ectopic expression of other cytokine receptors can support erythroid differentiation.(16,17) These receptors share many signaling pathways with the EpoR, including Stat5. Therefore, it is possible that they stimulate erythropoiesis because of common signaling mechanisms.
Homozygous deletion of the Stat5a and Stat5b genes has demonstrated that Stat5 is not required for normal RBC development in the adult animal.(14) In contrast, normal production of erythrocytes in the fetal liver does require Stat5a and Stat5b.(15) In the latter case, the Stat5 proteins appear to have an antiapoptotic function in the developing erythrocytes. These results are in agreement with a number of studies that have demonstrated a role for Stat5 in the induction of cytokine-responsive genes involved in growth control and protection from apoptosis.(27–31) Therefore, erythroid progenitors appear to have different requirements for Stat proteins during development. In this study, we demonstrated that EpoRs that activate Stat5 and Stat3 or that activate Stat3 in place of Stat5 support fetal liver CFU-E development. Therefore, Stat3 and Stat5 may share an antiapoptotic function. In support of this idea, Stat3 has been shown to play a critical role in IL-6-dependent T cell survival and proliferation.(32)
Recent work has shown that multiple tyrosine residues in the EpoR cytoplasmic region have overlapping and redundant roles in supporting erythropoiesis.(26,33) Therefore, the identity of the specific signal(s) regulating terminal erythroid development remains unclear. In light of the clinical relevance of Epo and the search for Epo mimetics, it is particularly important to determine if a specific signaling pathway is required or if multiple pathways can support RBC development. Additional studies using specifically engineered mutants of the EpoR in in vivo model systems are required to answer this question.
Acknowledgments
We thank Ann Hofbauer and Hong Lu for excellent technical support, Steve Magid and Tim Lee for assistance with plasmid constructions, and Joan Egrie and Steve Elliot (Amgen, Thousand Oaks, CA) for the generous gift of recombinant Epo. This work was supported by grants from the National Cancer Institute, National Institutes of Health (CA 77447), the Texas Higher Education Coordinating Board (15–120), and the Gillson Longenbaugh Foundation to S.S.W. and by a grant from the National Cancer Institute, National Institutes of Health (CA 75315) and an Established Investigator Award from the American Heart Association (9940116N) to G.D.L.
References
- 1.WU H, LIU X, JAENISCH R, LODISH HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 2.LIN CS, LIM SK, D’AGATI V, COSTANTINI F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 3.KIERAN MW, PERKINS AC, ORKIN SH, ZON LI. Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proc Natl Acad Sci USA. 1996;93:9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KRANTZ SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- 5.BAZAN JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WATOWICH SS, WU H, SOCOLOVSKY M, KLING-MULLER U, CONSTANTINESCU SN, LODISH HF. Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu Rev Cell Dev Biol. 1996;12:91–128. doi: 10.1146/annurev.cellbio.12.1.91. [DOI] [PubMed] [Google Scholar]
- 7.DAMEN JE, MUI AL-F, PUIL L, PAWSON T, KRYSTAL G. Phosphatidylinositol 3-kinase associates, via its src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993;81:3204–3210. [PubMed] [Google Scholar]
- 8.KLINGMULLER U, LORENZ U, CANTLEY LC, NEEL BG, LODISH HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of Jak2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 9.WITTHUHN BA, QUELLE FW, SILVENNOINEN O, YI T, TANG B, MIURA O, IHLE JN. Jak2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 10.DAMEN JE, WAKAO H, MIYAJIMA A, KROSL J, HUMPHRIES RK, CUTLER RL, KRYSTAL G. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GOBERT S, CHRETIEN S, GOUILLEUX F, MULLER O, PALLARD C, DUSANTER-FOURT I, GRONER B, LA-COMBE C, GISSELBRECHT S, MAYEUX P. Identification of tyrosine residues within the intracellular domain of the erythropoietin receptor crucial for Stat5 activation. EMBO J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]
- 12.DARNELL JE., Jr Stats and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 13.IHLE JN. Stats: Signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 14.TEGLUND S, McKAY C, SCHUETZ E, VAN DEURSEN JM, STRAVOPODIS D, WANG D, BROWN M, BODNER S, GROSVELD G, IHLE JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 15.SOCOLOVSKY M, FALLON AEJ, WANG S, BRUGNARA C, LODISH HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/−mice: a direct role for Stat5 in Bcl-XL induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 16.GOLDSMITH MA, MIKAMI A, YOU Y, LIU KD, THOMAS L, PHARR P, LONGMORE GD. Absence of cytokine receptor-dependent specificity in red blood cell differentiation in vivo. Proc Natl Acad Sci USA. 1998;95:7006–7011. doi: 10.1073/pnas.95.12.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SOCOLOVSKY M, DUSANTER-FOURT I, LODISH HF. The prolactin receptor and severly truncated erythropoietin receptors support differentiation of erythroid progenitors. J Biol Chem. 1997;272:14009–14012. doi: 10.1074/jbc.272.22.14009. [DOI] [PubMed] [Google Scholar]
- 18.WOOTEN DK, XIE X, BARTOS D, BUSCHE RA, LONG-MORE GD, WATOWICH SS. Cytokine signaling through Stat3 activates integrins, promotes adhesion, and induces growth arrest in the myeloid cell line 32D. J Biol Chem. 2000;275:26566–26575. doi: 10.1074/jbc.M003495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.STAHL N, FARRUGGELLA TJ, BOULTON TG, ZHONG Z, DARNELL JE, YANCOPOULOS GD. Choice of Stats and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 20.YOSHIMURA A, LONGMORE G, LODISH HF. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990;348:647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- 21.LONGMORE GD, LODISH HF. An activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: a cytokine receptor superfamily oncogene. Cell. 1991;67:1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- 22.LONGMORE GD, PHARR P, LODISH HF. A constitutively activated erythropoietin receptor stimulates proliferation and contributes to transformation of multipotent, committed nonerythroid and erythroid progenitor cells. Mol Cell Biol. 1994;14:2266–2277. doi: 10.1128/mcb.14.4.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WATOWICH SS, HILTON DJ, LODISH HF. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WATOWICH SS, LIU KD, XIE X, LAI SY, MIKAMI A, LONGMORE GD, GOLDSMITH MA. Oligomerization and scaffolding functions of the erythropoietin receptor cytoplasmic tail. J Biol Chem. 1999;274:5415–5421. doi: 10.1074/jbc.274.9.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PHARR PN, HANKINS D, HOFBAUER A, LODISH HF, LONGMORE GD. Expression of a constitutively active erythropoietin receptor in primary hematopoietic progenitors abrogates erythropoietin dependence and enhances erythroid colony-forming unit, erythroid burst-forming unit, and granulocyte/macrophage progenitor growth. Proc Natl Acad Sci USA. 1993;90:938–942. doi: 10.1073/pnas.90.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LONGMORE GD, YOU Y, MOLDEN J, LIU KD, MIKAMI A, LAI SY, PHARR P, GOLDSMITH MA. Redundant and selective roles for erythropoietin receptor tyrosines in erythropoiesis in vivo. Blood. 1998;91:870–878. [PubMed] [Google Scholar]
- 27.MUI AL-F, WAKAO H, KINOSHITA T, KITAMURA T, MIYAJIMA A. Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 28.MORIGGL R, TOPHAM DJ, TEGLUND S, SEXL V, McKAY C, WANG D, HOFFMEYER A, VAN DEURSEN J, SANGSTER MY, BUNTING KD, GROSVELD GC, IHLE JN. Stat5 is required for IL-2-induced cell cycle progression in peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 29.MATSUMURA I, KITAMURA T, WAKAO H, TANAKA H, HASHIMOTO K, ALBANESE C, DOWNWARD J, PESTELL RG, KANAKURA Y. Transcriptional regulation of the cyclin D1 promoter by Stat5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ZAMORANO J, WANG HY, WANG R, SHI Y, LONG-MORE GD, KEEGAN AD. Regulation of cell growth by IL-2: role of Stat5 in protection from apoptosis but not in cell cycle progression. J Immunol. 1998;160:3502–3512. [PubMed] [Google Scholar]
- 31.NOSAKA T, KAWASHIMA T, MISAWA K, IKUTA K, MUI AL-F, KITAMURA T. Stat5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TAKEDA K, KAISHO T, YOSHIDA N, TAKEDA J, KISHI-MOTO T, AKIRA S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 33.KLINGMULLER U, WU H, HSIAO J, TOKER A, DUCK-WORTH BC, CANTLEY LC, LODISH HF. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci USA. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]


