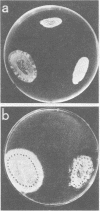
Abstract
A screening technique for microbial proteases, the thin-layer enzyme assay cultivation technique, was developed. The inner surface of a polystyrene petri dish was coated with protein and then covered with a culture agar medium. The enzymes, produced during growth of the microorganisms, reach the protein-coated surface by diffusion in the agar. Degradation of the protein was visualized by condensation of water vapor on the surface after removal of the agar medium. The wettability of the enzyme-affected protein-coated polystyrene surface was decreased compared with the unaffected protein surface. Enzyme substrates used were fibrinogen, immunoglobulin G, egg albumin, human serum albumin, bovine serum albumin, hemoglobin, mucin, and gelatin. It was possible to use a variety of culture agar media, nonselective as well as selective, in the assay. The technique provides a sensitive, convenient, and inexpensive method for screening various microbial proteases. In addition, the technique can be used for screening proteolytic enzyme activity of specific microbial species in a mixed microbial sample as well as for studies of factors that influence the cultivation conditions for protease production and activity.
Full text
PDF

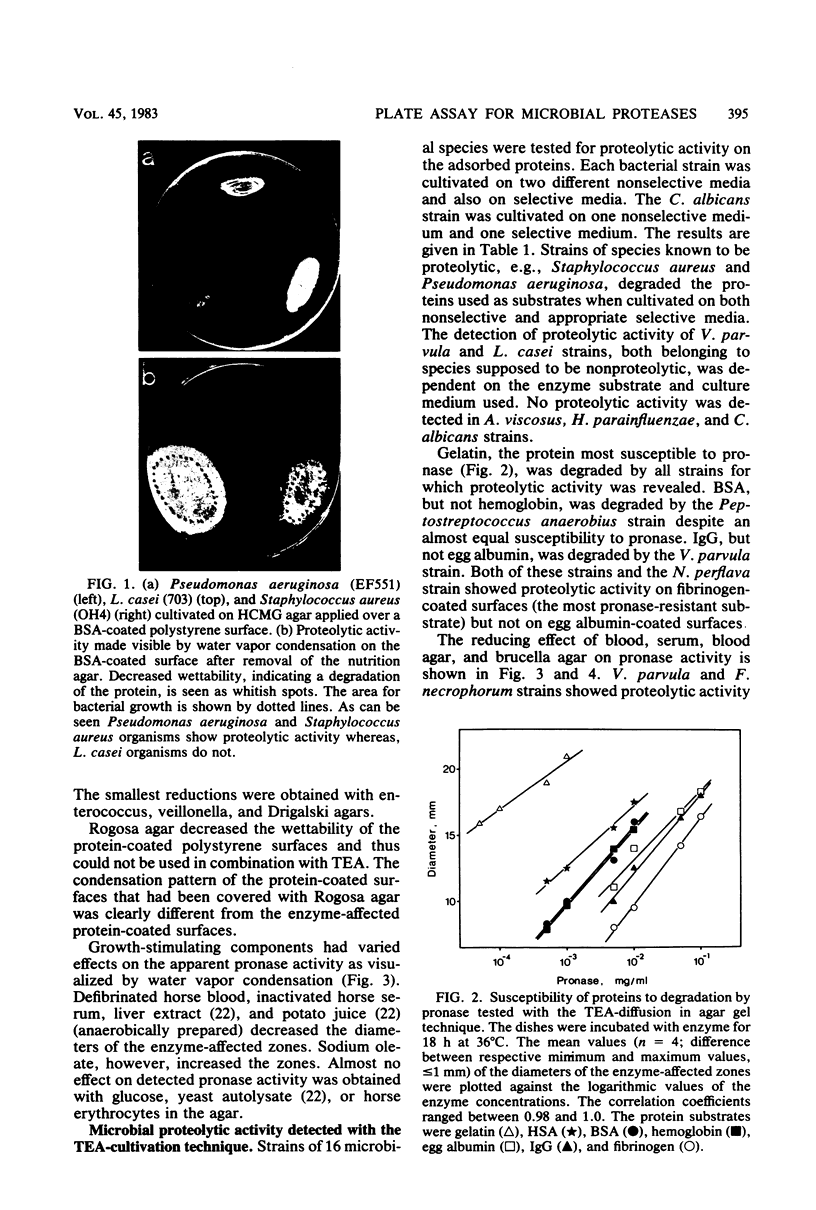
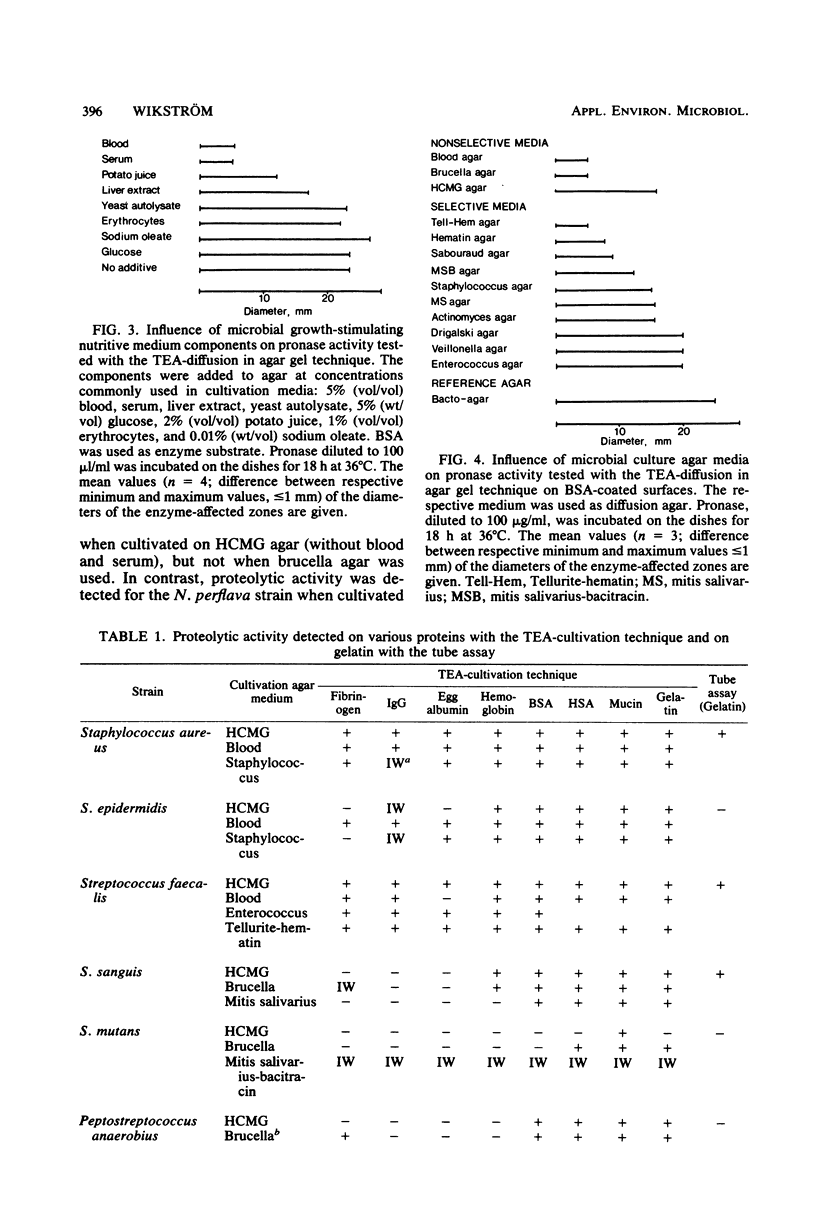
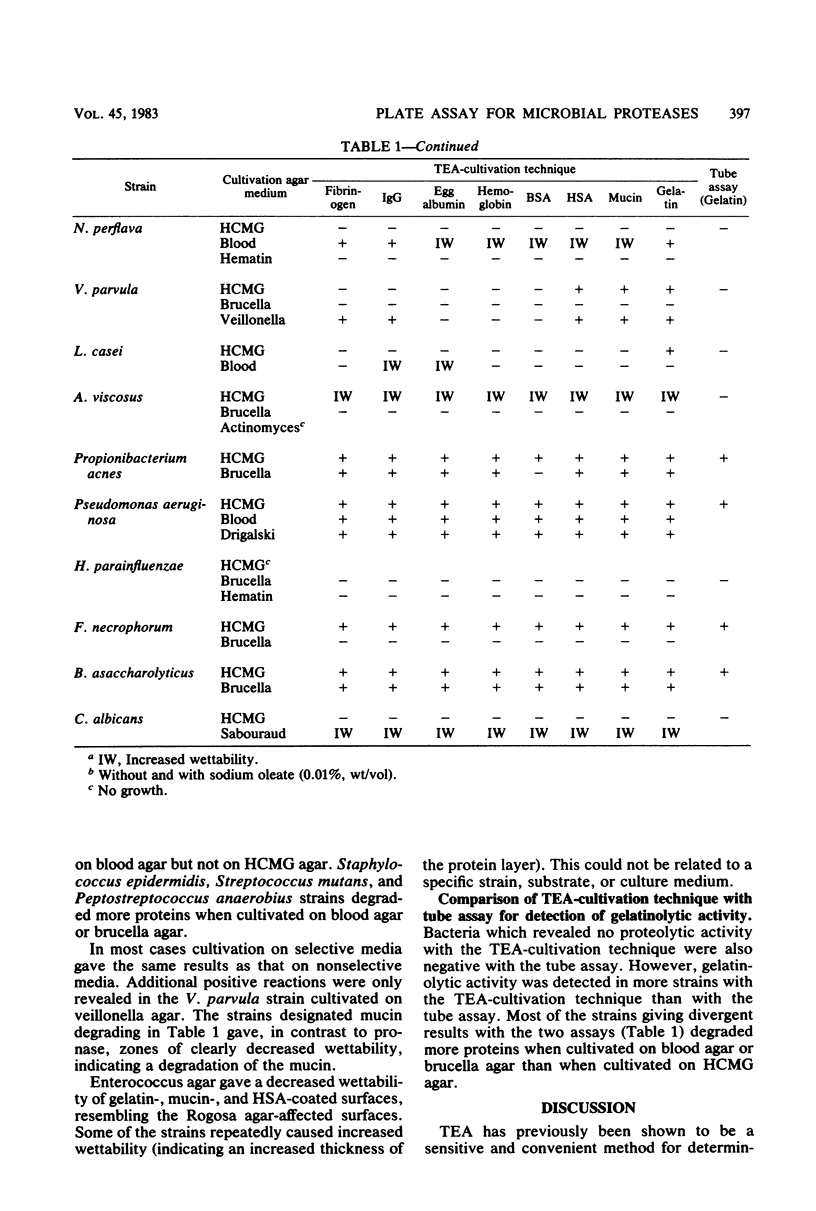



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DURHAM N. N., McPHERSON D. L. Influence of extraneous carbon sources on biosynthesis de novo of bacterial enzymes. J Bacteriol. 1960 Jul;80:7–13. doi: 10.1128/jb.80.1.7-13.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Elwing H., Nilsson L., Ouchterlony O. Visualization principles in thin-layer immunoassays (TIA) on plastic surfaces. Int Arch Allergy Appl Immunol. 1976;51(6):757–762. doi: 10.1159/000231654. [DOI] [PubMed] [Google Scholar]
- Elwing H. Water wettability of antigen and antigen-antibody layers on solid surfaces studied by the contact angle measurement technique. FEBS Lett. 1980 Jul 28;116(2):239–242. doi: 10.1016/0014-5793(80)80653-3. [DOI] [PubMed] [Google Scholar]
- Fabricius L., Dahlén G., Holm S. E., Möller A. J. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res. 1982 Jun;90(3):200–206. doi: 10.1111/j.1600-0722.1982.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Fossum K. Proteolytic enzymes and biological inhibitors. II. Naturally occurring inhibitors in sera from different species and their effect upon proteolytic enzymes of various origin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):605–618. [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M., Johnson G. L. Proteolytic activity of Candida albicans: action on human salivary proteins. Infect Immun. 1978 Dec;22(3):861–866. doi: 10.1128/iai.22.3.861-866.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. New medium for isolation of Actinomyces viscosus and Actinomyces naeslundii from dental plaque. J Clin Microbiol. 1978 Jun;7(6):514–518. doi: 10.1128/jcm.7.6.514-518.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Möller A. J. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr. 1966 Dec 20;74(5 Suppl):1–380. [PubMed] [Google Scholar]
- Norén I., Ramström G., Wallén P. Fibrin plate method with reagents purified by affinity chromatography and its use for determination of fibrinolytic and other proteolytic activity in saliva, bile and plasma. Haemostasis. 1975;4(2):110–124. doi: 10.1159/000214094. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., FITZGERALD R. J., MACKINTOSH M. E., BEAMAN A. J. Improved medium for selective isolation of Veillonella. J Bacteriol. 1958 Oct;76(4):455–456. doi: 10.1128/jb.76.4.455-456.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. L., Jr, GOODNER K. Detection of bacterial gelatinases by gelatin-agar plate methods. J Bacteriol. 1958 Dec;76(6):662–665. doi: 10.1128/jb.76.6.662-665.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samloff I. M., Kleinman M. S. A radial diffusion assay for pepsinogen and pepsin. Gastroenterology. 1969 Jan;56(1):30–34. [PubMed] [Google Scholar]
- Santarius K., Ryan C. Radial diffusion as a sensitive method for screening endopeptidase activity in plant extracts. Anal Biochem. 1977 Jan;77(1):1–9. doi: 10.1016/0003-2697(77)90283-4. [DOI] [PubMed] [Google Scholar]
- Schill W. B., Schumacher G. F. Radial diffusion in gel for micro determination of enzymes. I. Muramidase, alpha-amylase, DNase 1, RNase A, acid phosphatase, and alkaline phosphatase. Anal Biochem. 1972 Apr;46(2):502–533. doi: 10.1016/0003-2697(72)90324-7. [DOI] [PubMed] [Google Scholar]
- Schumacher G. F., Schill W. B. Radial diffusion in gel for micro determination of enzymes. II. Plasminogen activator, elastase, and nonspecific proteases. Anal Biochem. 1972 Jul;48(1):9–26. doi: 10.1016/0003-2697(72)90165-0. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen E. K., Hentges D. J. Hydrolytic enzymes of anaerobic bacteria isolated from human infections. J Clin Microbiol. 1981 Aug;14(2):153–156. doi: 10.1128/jcm.14.2.153-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M., Elwing H., Linde A. Determination of proteolytic activity: a sensitive and simple assay utilizing substrate adsorbed to a plastic surface and radial diffusion in gel. Anal Biochem. 1981 Dec;118(2):240–246. doi: 10.1016/0003-2697(81)90185-8. [DOI] [PubMed] [Google Scholar]