Abstract
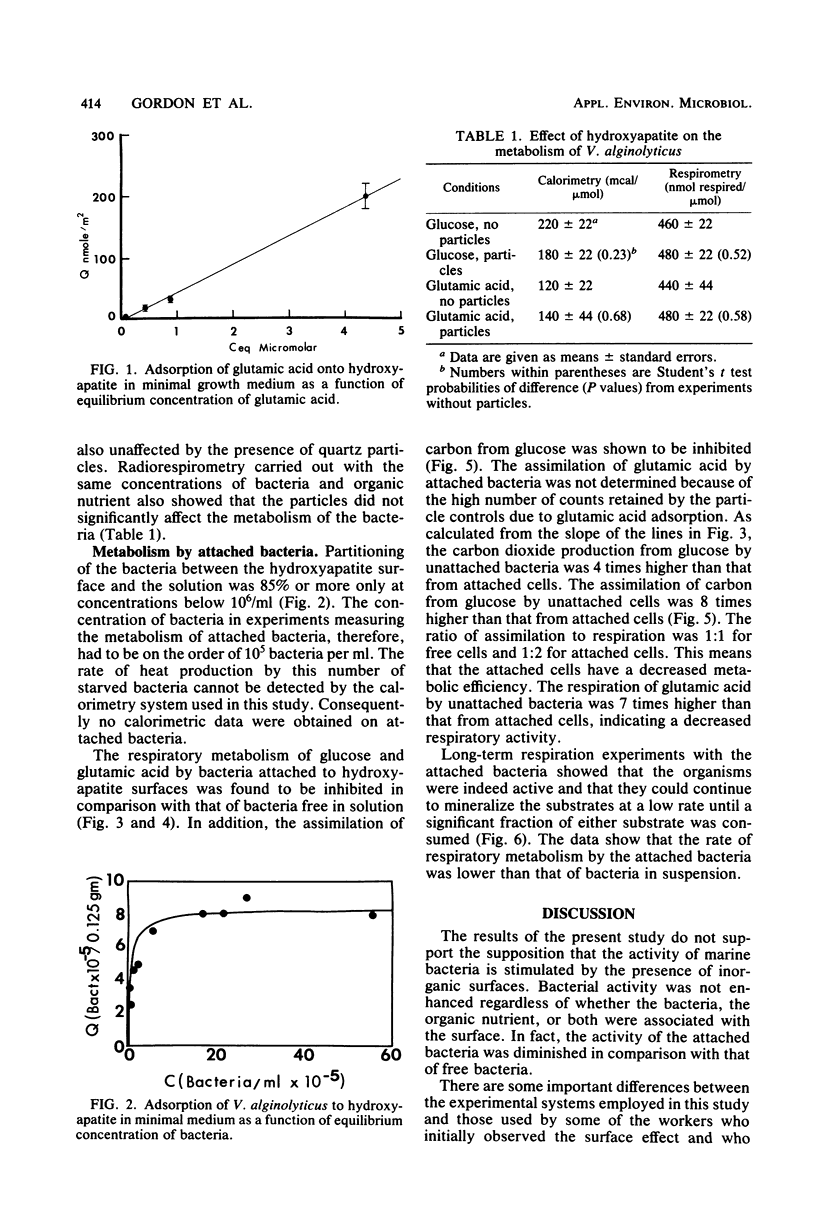
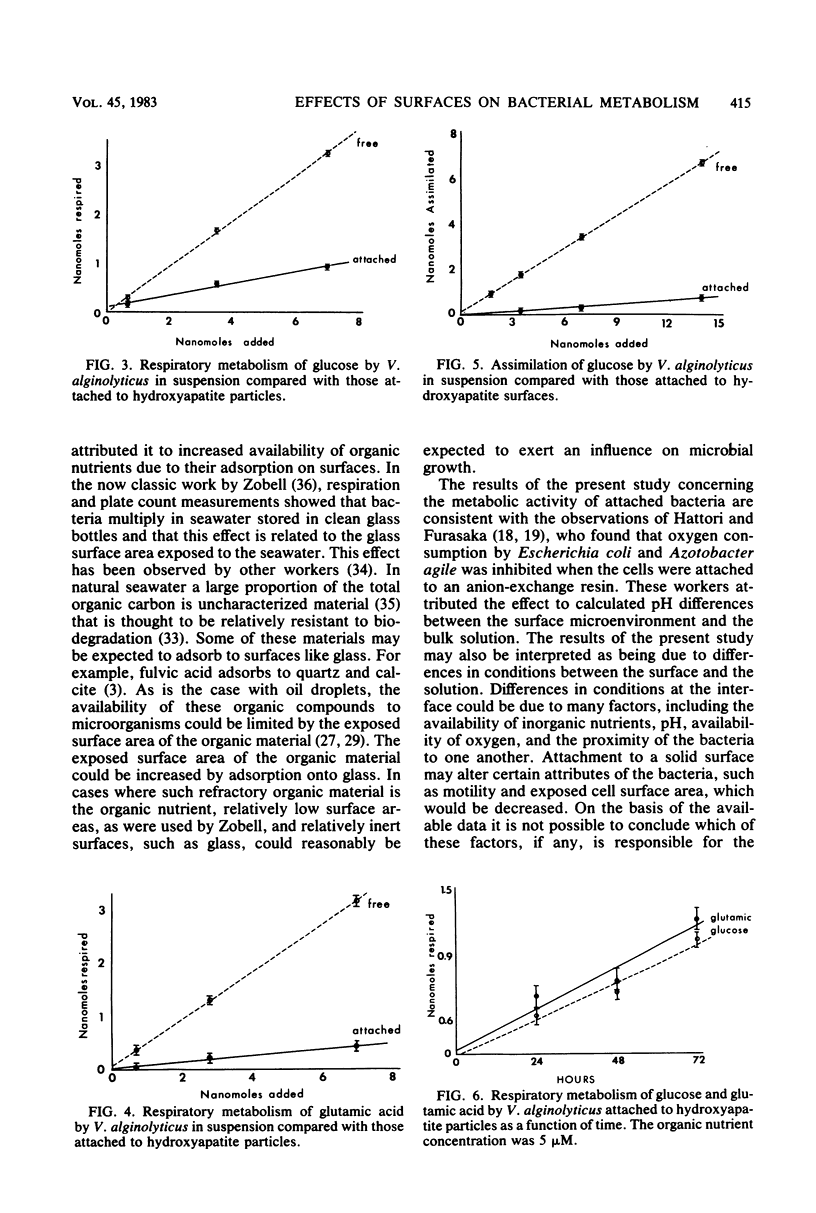
Measurements were made of adsorption of a periphytic marine bacterium, glucose, and glutamic acid to inorganic particles in seawater and defined bacterial growth medium. Measurements of the metabolism of bacteria were made in the presence and absence of particles by microcalorimetry and radiorespirometry. It was found that hydroxyapatite adsorbs glutamic acid, but not glucose, from the experimental medium. It was also found that hydroxyapatite adsorbs essentially all of the bacteria from the medium when the bacterial concentration is approximately 6 × 105 bacteria per ml. If the bacterial concentration is approximately 6 × 107, then only a small fraction of cells become attached. It was therefore possible to select bacterial concentrations and organic nutrients so that bacterial attachment, organic nutrient adsorption, or both would occur in different experiments. In this experimental system the metabolism by attached and nonattached bacteria of adsorbing and nonadsorbing organic nutrients was measured. The results show that bacterial activity in this model system was not enhanced by the particles, regardless of whether the bacteria, the organic nutrient, or both were associated with the surface. In fact, the respiratory activity of the attached bacteria was diminished in comparison with that of free bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. R., Albright L. J. Attached and free-floating bacteria in a diverse selection of water bodies. Appl Environ Microbiol. 1982 Jun;43(6):1227–1237. doi: 10.1128/aem.43.6.1227-1237.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M., Loeb G. I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol. 1979 Jan;37(1):67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Gerchakov S. M., Udey L. R. The effect of polarization on the attachment of marine bacteria to copper and platinum surfaces. Can J Microbiol. 1981 Jul;27(7):698–703. doi: 10.1139/m81-108. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Millero F. J., Gerchakov S. M. Microcalorimetric Measurements of Glucose Metabolism by Marine Bacterium Vibrio alginolyticus. Appl Environ Microbiol. 1982 Nov;44(5):1102–1109. doi: 10.1128/aem.44.5.1102-1109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTORI T., FURUSAKA C. Chemical activities of Azotobacter agile adsorbed on a resin. J Biochem. 1961 Oct;50:312–315. doi: 10.1093/oxfordjournals.jbchem.a127450. [DOI] [PubMed] [Google Scholar]
- Heukelekian H., Heller A. Relation between Food Concentration and Surface for Bacterial Growth. J Bacteriol. 1940 Oct;40(4):547–558. doi: 10.1128/jb.40.4.547-558.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Colwell R. R. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl Microbiol. 1975 Feb;29(2):269–274. doi: 10.1128/am.29.2.269-274.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Mitchell R. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982 Jan;43(1):200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange W. Speculations on a possible essential function of the gelatinous sheath of blue-green algae. Can J Microbiol. 1976 Aug;22(8):1181–1185. doi: 10.1139/m76-171. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981 Oct;148(1):51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman S. A., Carey C. L. Decomposition of Organic Matter in Sea Water by Bacteria: I. Bacterial Multiplication in Stored Sea Water. J Bacteriol. 1935 May;29(5):531–543. doi: 10.1128/jb.29.5.531-543.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


