Abstract
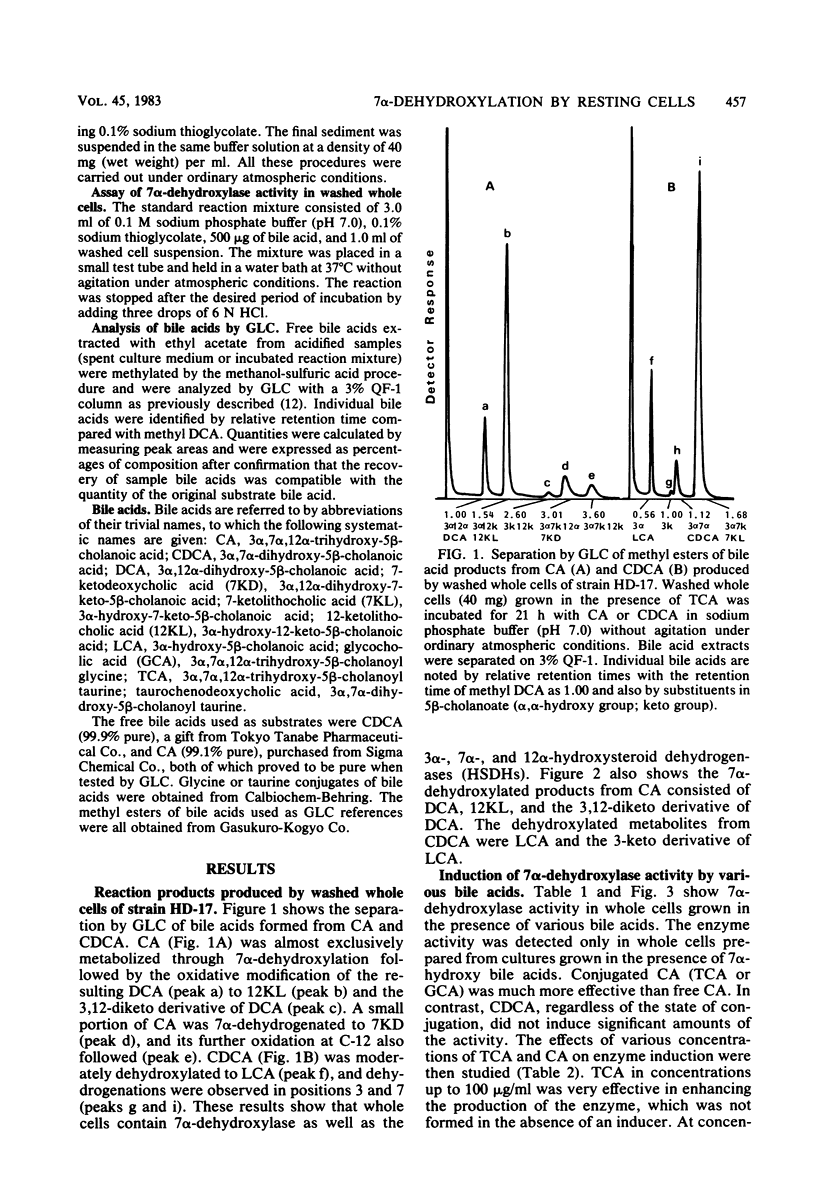
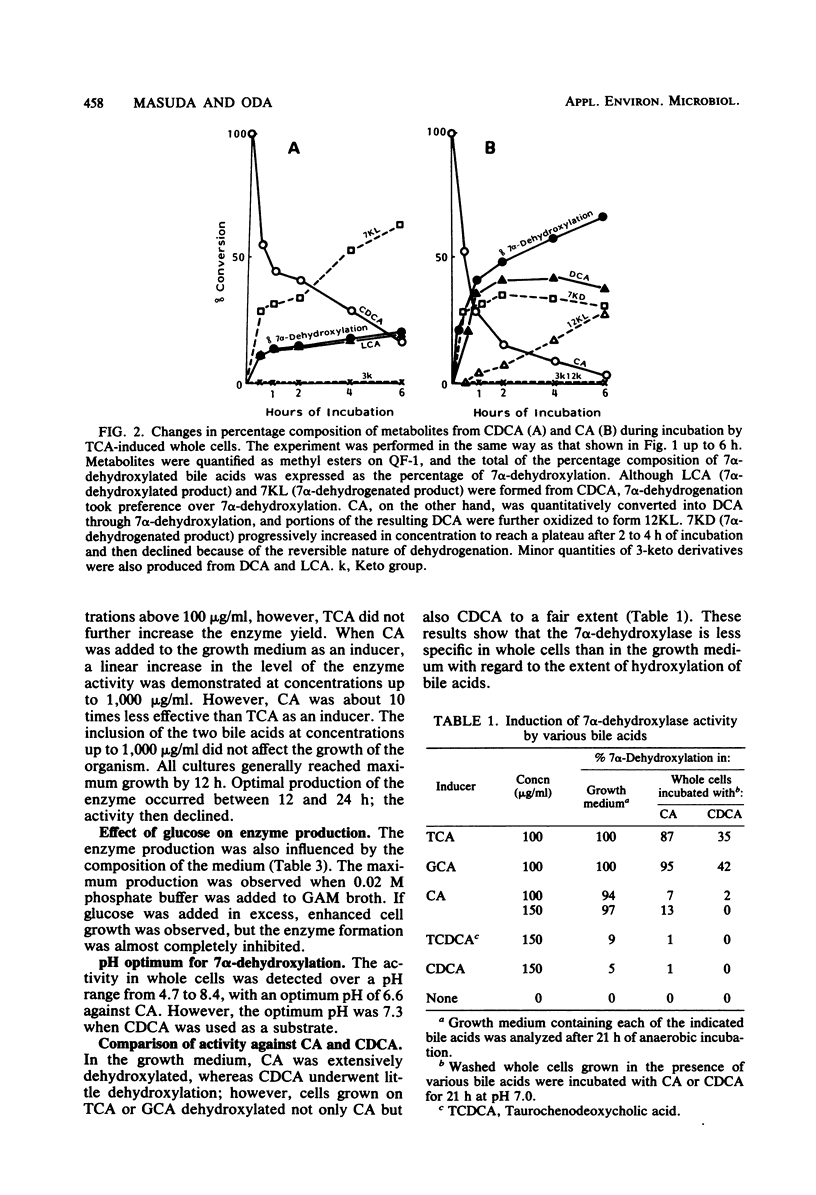
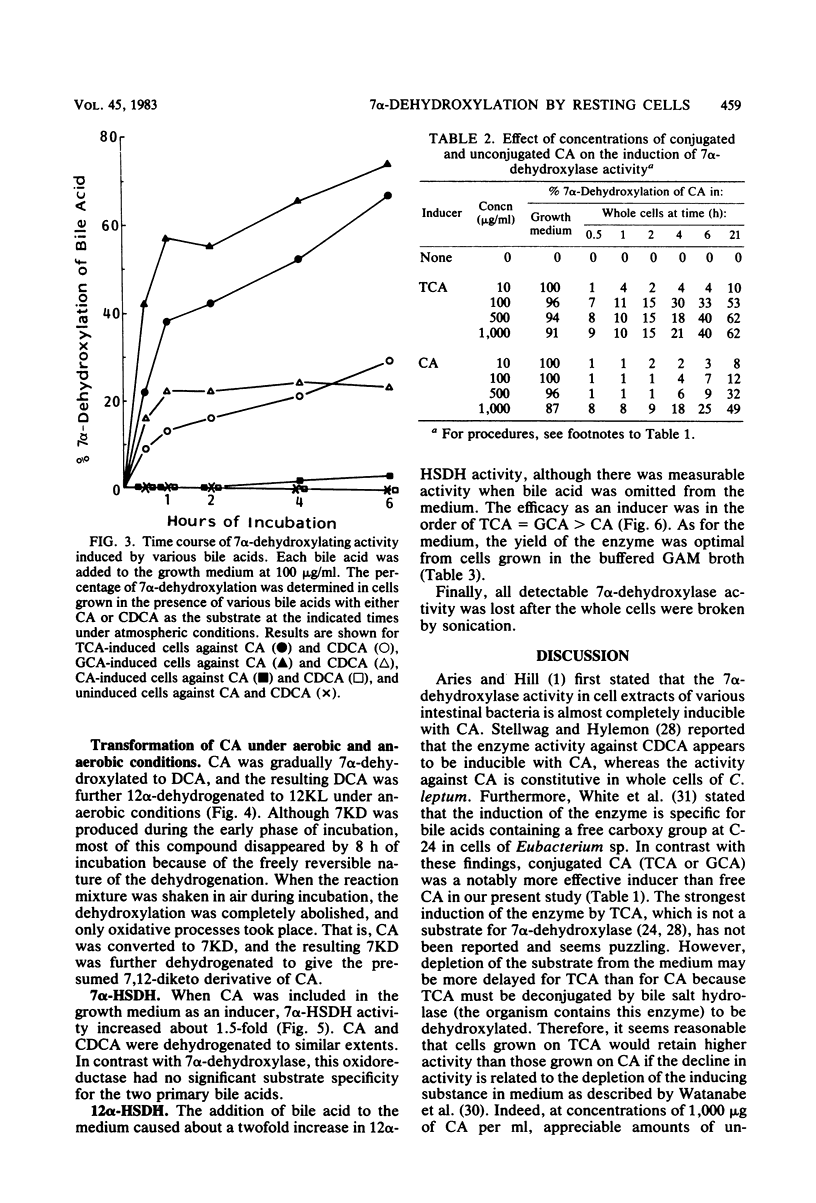
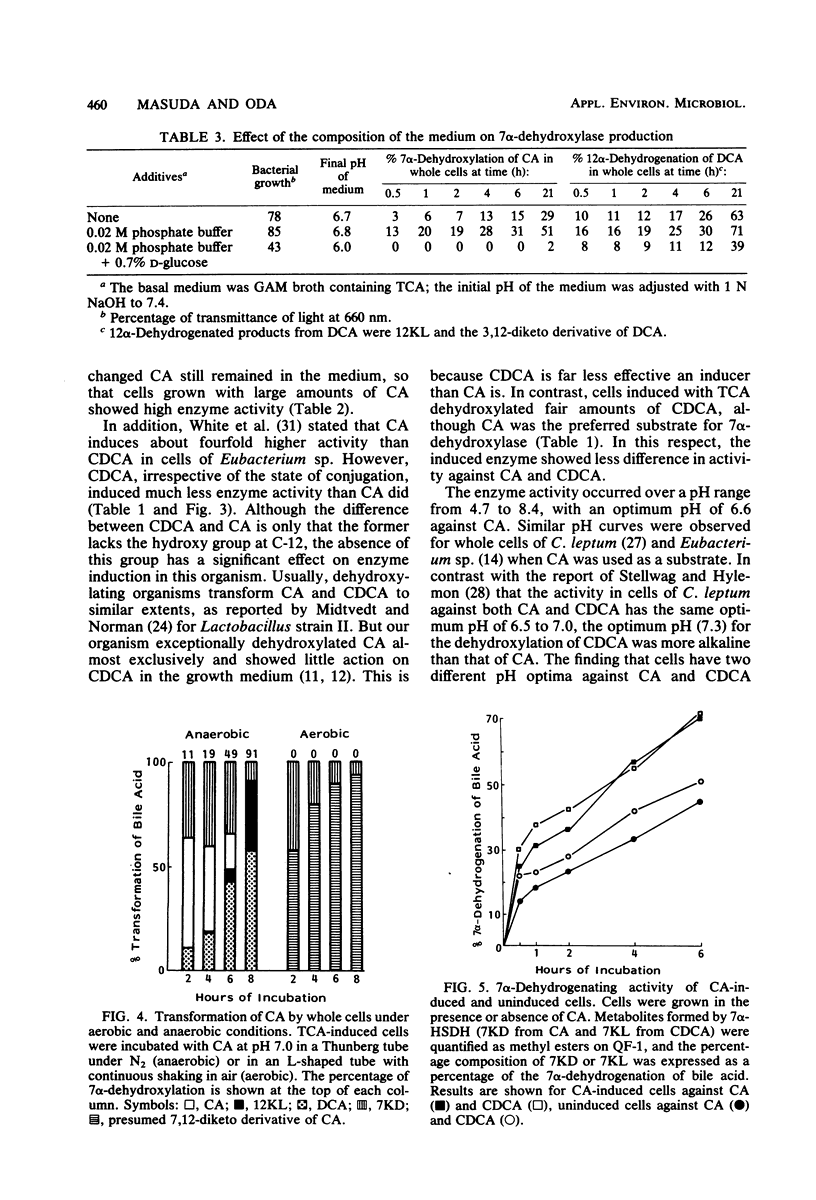
Transformation of bile acids by washed whole cells of strain HD-17, an unidentified gram-positive anaerobic bacterium isolated from human feces, was studied. 7 alpha-Dehydroxylase was produced only during adaptive growth on medium containing 7 alpha-hydroxy bile acids. Both the extent of hydroxylation and the state of conjugation of the bile acids had marked effects on the induction of the enzyme, and the order of the enzyme induction was conjugated cholic acid much greater than cholic acid greater than taurochenodeoxycholic acid greater than or equal to chenodeoxycholic acid. The addition of excess glucose to the growth medium appreciably reduced the enzyme level. The induced enzyme required strict anaerobic conditions for activity and had an optimal pH range of 6.5 to 7.5. In contrast with the induction of the enzyme, the induced enzyme showed a low degree of substrate specificity between cholic acid and chenodeoxycholic acid, with some preference for the former. In addition, the organism contained 3 alpha-, 7 alpha-, and 12 alpha-hydroxysteroid dehydrogenases, and the addition of bile acids to the medium somewhat enhanced the production of the oxidoreductases. The dehydrogenations were obviously stimulated by oxygen as a terminal electron acceptor. The organism also contained bile salt hydrolase.
Full text
PDF
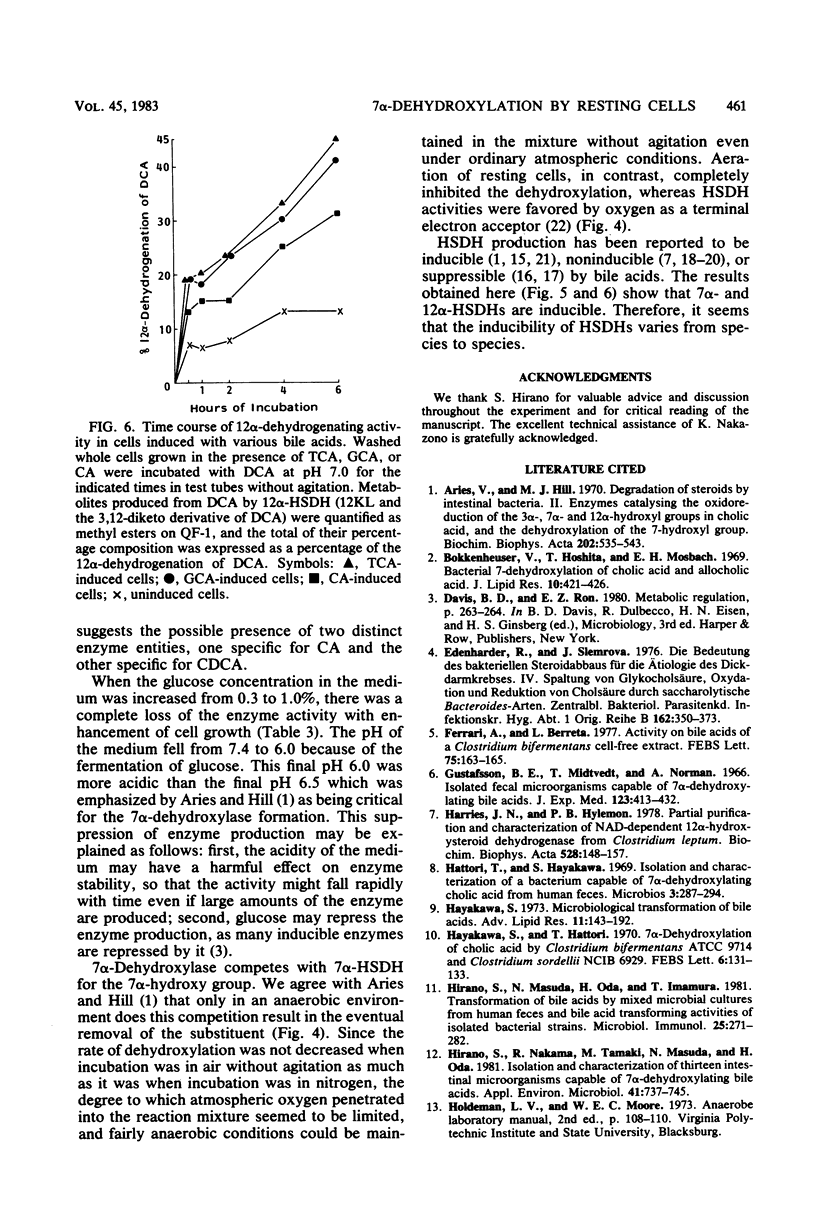

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. II. Enzymes catalysing the oxidoreduction of the 3 alpha-, 7 alpha- and 12 alpha-hydroxyl groups in cholic acid, and the dehydroxylation of the 7-hydroxyl group. Biochim Biophys Acta. 1970 May 5;202(3):535–543. doi: 10.1016/0005-2760(70)90124-4. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser V., Hoshita T., Mosbach E. H. Bacterial 7-dehydroxylation of cholic acid and allocholic acid. J Lipid Res. 1969 Jul;10(4):421–426. [PubMed] [Google Scholar]
- Edenharder R., Slemrova J. Die Bedeutung des bakteriellen Steroidabbaus für die Atiologie des Dickdarmkrebses. IV. Spaltung von Glykocholsäure, Oxydation und Reduktion von Cholsäure durch saccharolytische Bacteroides-Arten. Zentralbl Bakteriol Orig B. 1976 Jul;162(3-4):350–373. [PubMed] [Google Scholar]
- Ferrari A., Beretta L. Activity on bile acids of a Clostridium bifermentans cell-free extract. FEBS Lett. 1977 Mar 15;75(1):163–165. doi: 10.1016/0014-5793(77)80076-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. E., Midtvedt T., Norman A. Isolated fecal microorganisms capable of 7-alpha-dehydroxylating bile acids. J Exp Med. 1966 Feb 1;123(2):413–432. doi: 10.1084/jem.123.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. N., Hylemon P. B. Partial purification and characterization of NADP-dependent 12alpha-hydroxysteroid dehydrogenase from Clostridium leptum. Biochim Biophys Acta. 1978 Jan 27;528(1):148–157. doi: 10.1016/0005-2760(78)90060-7. [DOI] [PubMed] [Google Scholar]
- Hayakawa S., Hattori T. 7alpha-dehydroxylation of cholic acid by Clostridium bifermentans strain ATCC 9714 and Clostridium sordellii strain NCIB 6929. FEBS Lett. 1970 Jan 26;6(2):131–133. doi: 10.1016/0014-5793(70)80020-5. [DOI] [PubMed] [Google Scholar]
- Hayakawa S. Microbiological transformation of bile acids. Adv Lipid Res. 1973;11:143–192. doi: 10.1016/b978-0-12-024911-4.50011-8. [DOI] [PubMed] [Google Scholar]
- Hirano S., Masuda N., Oda H., Imamura T. Transformation of bile acids by mixed microbial cultures from human feces and bile acid transforming activities of isolated bacterial strains. Microbiol Immunol. 1981;25(3):271–282. doi: 10.1111/j.1348-0421.1981.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Hirano S., Nakama R., Tamaki M., Masuda N., Oda H. Isolation and characterization of thirteen intestinal microorganisms capable of 7 alpha-dehydroxylating bile acids. Appl Environ Microbiol. 1981 Mar;41(3):737–745. doi: 10.1128/aem.41.3.737-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Cacciapuoti A. F., White B. A., Whitehead T. R., Fricke R. J. 7 alpha-Dehydroxylation of cholic acid by cell extracts of Eubacterium species V.P.I. 12708. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2507–2510. doi: 10.1093/ajcn/33.11.2507. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Sherrod J. A. Multiple forms of 7-alpha-hydroxysteroid dehydrogenase in selected strains of Bacteroides fragilis. J Bacteriol. 1975 May;122(2):418–424. doi: 10.1128/jb.122.2.418-424.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS P. I., TALALAY P. Induction and purification of alpha- and beta-hydroxysteroid dehydrogenases. J Biol Chem. 1956 Feb;218(2):661–674. [PubMed] [Google Scholar]
- MacDonald I. A., Mahony D. E., Jellet J. F., Meier C. E. NAD-dependent 3alpha- and 12alpha-hydroxysteroid dehydrogenase activities from Eubacterium lentum ATCC no. 25559. Biochim Biophys Acta. 1977 Dec 21;489(3):466–476. doi: 10.1016/0005-2760(77)90167-9. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Jellett J. F., Mahony D. E. 12alpha-Hydroxysteroid dehydrogenase from Clostridium group P strain C48-50 ATCC No. 29733: partial purification and characterization. J Lipid Res. 1979 Feb;20(2):234–239. [PubMed] [Google Scholar]
- Macdonald I. A., Meier E. C., Mahony D. E., Costain G. A. 3alpha-, 7alpha- and 12alpha-hydroxysteroid dehydrogenase activities from Clostridium perfringens. Biochim Biophys Acta. 1976 Nov 19;450(2):142–153. doi: 10.1016/0005-2760(76)90086-2. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E. 7Alpha-hydroxysteroid dehydrogenase from Escherichia coli B: preliminary studies. Biochim Biophys Acta. 1973 Jun 6;309(2):243–253. doi: 10.1016/0005-2744(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Macdonald I. A., Williams C. N., Mahony D. E., Christie W. M. NAD- and NADP-dependent 7alpha-hydroxysteroid dehydrogenases from bacteroides fragilis. Biochim Biophys Acta. 1975 Mar 28;384(1):12–24. doi: 10.1016/0005-2744(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Masuda N. Deconjugation of bile salts by Bacteroids and Clostridium. Microbiol Immunol. 1981;25(1):1–11. doi: 10.1111/j.1348-0421.1981.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Midtvedt T., Norman A. Parameters in 7-alpha-dehydroxylation of bile acids by anaerobic lactobacilli. Acta Pathol Microbiol Scand. 1968;72(2):313–329. doi: 10.1111/j.1699-0463.1968.tb01345.x. [DOI] [PubMed] [Google Scholar]
- NORMAN A., SHORB M. S. In vitro formation of deoxycholic and lithocholic acid by human intestinal microorganisms. Proc Soc Exp Biol Med. 1962 Jul;110:552–555. doi: 10.3181/00379727-110-27577. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979 Mar;20(3):325–333. [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. Characterization of 7-alpha-dehydroxylase in Clostridium leptum. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S243–S247. doi: 10.1093/ajcn/31.10.S243. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Phillips K., Watanabe H. Induction of steroid-binding activity in Pseudomonas testosteroni. J Steroid Biochem. 1973 Nov;4(6):623–632. doi: 10.1016/0022-4731(73)90037-x. [DOI] [PubMed] [Google Scholar]
- White B. A., Lipsky R. L., Fricke R. J., Hylemon P. B. Bile acid induction specificity of 7 alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids. 1980 Jan;35(1):103–109. doi: 10.1016/0039-128x(80)90115-4. [DOI] [PubMed] [Google Scholar]


