Abstract
In this report, we define requirements for the successful translocation and functional maturation of the adhesin P1 of Streptococcus mutans. Conformational epitopes recognized by anti-P1 monoclonal antibodies (MAbs) were further characterized, thus facilitating the use of particular MAbs as tools to monitor the locations of various forms of the protein. We show that correct localization of P1 is dependent on structural features of the molecule itself, including a requisite A region-P region intramolecular interaction that occurs within the cell prior to secretion. P1 also was shown to be affected by several members of the protein-folding-secretion-turnover apparatus. It does not achieve a fully functional form in the absence of the trigger factor PPIase homolog RopA, and its translocation is delayed when DnaK levels are limited. In addition, dnaK message levels are differentially altered in the presence of P1 lacking the alanine-rich compared to the proline-rich repeat domains. Lastly, nonsecreted P1 lacking the P region accumulates within the cell in the absence of htrA, implying an intracellular HtrA protease function in the degradation and turnover of this particular internal-deletion polypeptide. However, the opposite effect is seen for full-length P1, suggesting a sensing mechanism and substrate-dependent alteration in HtrA's function and effect that is consistent with its known ability to switch between chaperone and protease, depending on environmental perturbations.
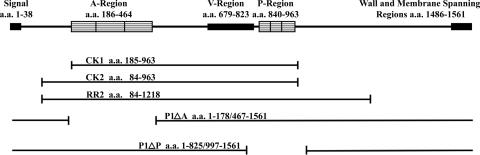
Streptococcus mutans is an oral bacterium that is a major etiologic agent of dental caries (19). The multidomain (Mr, ∼185,000) wall-associated multifunctional fibrillar cell surface protein P1 (15), encoded by spaP (28, 33), was originally identified as antigen (Ag) I/II (59). It is also called PAc (48), encoded by pac (49), and is widely understood to enable attachment of S. mutans to the acquired salivary pellicle on teeth. Ag I/II family proteins are expressed by most oral streptococci and mediate interactions with salivary constituents; host cell matrix proteins, such as fibronectin, fibrinogen, and collagen; and other oral bacteria (reviewed in reference 26). They share architectural features with a broad category of surface adhesins prevalent among streptococci and staphylococci called microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), whose functions are dependent on complex structural elements (63). P1 contains a 38-residue amino-terminal signal sequence, a series of three 82-residue alanine-rich tandem repeats (A region); a 144-residue variable (V) region, where 20/36 amino acid differences between P1 and PAc are clustered (4); a central proline-rich (P) region, comprised of three 39-residue tandem repeats; and carboxy-terminal membrane- and wall-spanning regions, including the LPXTG motif characteristic of wall-anchored sortase substrates (14, 66). A schematic representation of the molecule and derivatives evaluated in this study is shown in Fig. 1.
FIG. 1.
Schematic representation of the primary sequence of the S. mutans cell surface-localized adhesin P1. Relevant structural domains are illustrated. Recombinant P1 polypeptides and internal-deletion constructs used in the experiments are indicated.
Several regions have been implicated in the ligand binding activities of Ag I/II family polypeptides. Brady et al. (7) demonstrated that P1 possesses multiple sites contributing to its interaction with the high-molecular-weight salivary glycoprotein known as salivary agglutinin, now known to represent the human salivary scavenger protein gp340 (54). Later, Scatchard analysis of Ag I/II binding to saliva-coated hydroxyapatite showed the binding to be mediated by two sites (18), although studies have also documented binding of isolated recombinant peptide fragments to salivary components (11, 29, 43, 44). Loimaranta et al. (36) have studied the gp340 interaction with multiple streptococcal species, and like Brady et al. (7), have observed different recognition properties with fluid phase than with immobilized agglutinin. X-ray crystallography of the central portion of P1, including the variable region, revealed a flexible beta-sandwich placing the A region and P region into close proximity (67). Several studies have now shown that the A and P regions of P1 contribute to complex conformational epitopes recognized by anti-P1 monoclonal antibodies (MAbs), some of which can be reconstituted by the interaction of recombinant A and P region-containing polypeptides (41, 57, 58, 60, 69).
In-frame deletion of either the A region (P1ΔA) or the P region (P1ΔP) of P1 results in a loss of surface-localized P1 on S. mutans, even when N-terminal signal and C-terminal anchor sequences are not altered (5, 60). Internally deleted molecules are not released into culture supernatants and are barely detectable in S. mutans cytoplasmic fractions, despite the presence of wild-type levels of spaP mRNA. Presumably, the altered polypeptides are degraded intracellularly and not secreted. When P1ΔA and P1ΔP polypeptides are expressed in Escherichia coli, they lose reactivity with an overlapping subset of anti-P1 MAbs that recognize full-length P1 but do not recognize either the A region or P region alone. Using E. coli as a model for secretion, full-length P1 is translocated across the inner membrane. This occurs even in a SecB-negative mutant background, as would be expected given the absence of a SecB homolog in streptococci; however, neither P1ΔA nor P1ΔP is secreted into the E. coli periplasm (60).
The mechanism of secretion and maturation of streptococcal surface proteins, such as P1, is not well understood. Current evidence suggests that sortase-anchored proteins are translocated via the Sec translocon (40). The presence of P1 on the cell surface (L. J. Brady, unpublished data) in an S. mutans mutant devoid of the cotranslational signal recognition pathway (20) also argues that it is secreted in a posttranslational manner via the general secretion pathway. A large protein of 1,561 residues would almost certainly require interactions with chaperones to ensure proper targeting and prevent aggregation while transiting to the cell surface. The goals of the study described in this report were to evaluate further the nature of the A region-P region interaction of P1, to determine whether an interaction between the A and P regions is requisite for surface localization of P1 in S. mutans, and to establish whether such an interaction occurs within the cell prior to secretion. In addition, the contributions of RopA, DnaK, and HtrA to P1 quality control, surface localization, and functional maturation were explored using mutant strains of S. mutans in which expression of the chaperone or protease of interest had been eliminated or substantially down-regulated.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study and their sources are listed in Table 1. S. mutans strains were grown in Todd-Hewitt broth (BBL, Cockeysville, MD) supplemented with 0.3% (wt/vol) yeast extract (THYE). E. coli strains DH5α, JM109, and TOP10 were grown at 37°C with vigorous shaking in Luria-Bertani broth supplemented with 50 to 100 μg ampicillin ml−1 or 50 μg kanamycin ml−1 as necessary.
TABLE 1.
Strains and plasmids
| Strains and plasmids | Relevant feature(s) | Reference or source |
|---|---|---|
| Strains | ||
| S. mutans | ||
| NG8 | Wild-type serotype c strain | 31 |
| UA159 | Wild-type serotype c strain | 2 |
| PC3370 | NG8 (ΔspaP); Tetr | 10 |
| AH3370 | UA159 (ΔspaP); Tetr | This study |
| PC3370A | PC3370 harboring pDL289 (vector only); Tetr Kanr | 5 |
| PC3370B | PC3370 harboring pMAJJ8 (P1ΔP); Tetr Kanr | 5 |
| PC3370C | PC3370 harboring pMAD (full-length P1); Tetr Kanr | 5 |
| PC3370D | PC3370 harboring pTS21 (P1ΔA); Tetr Kanr | 60 |
| PC967 | PC3370 harboring pPC967 (P1ΔP with reconstituted P region); Tetr Kanr | This study |
| TW90 | UA159 (ΔropA); Ermr | 71 |
| SM12 | UA159 (down-regulated dnaK); Kanr | 35 |
| SAB2 | UA159 (htrA); Ermr | 1 |
| E. coli | ||
| Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 endA1 araΔ139 Δ(ara, leu)7697 galU galK λ−rpsL(Strr) nupG | Invitrogen (Carlsbad, CA) |
| DH5α | F′ F80dlacΔ (lacZ) M15ΔendA1 hsdR17 supE44 thi1 recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 | Bio-Rad (Hercules, CA) |
| MC1061 | Δ(araA-leu)7697 araD139 Δ(codB-lac)3 galE15 galK16 mcrA0 relA1 rpsl150 spoT1 mcrB9999 hsdR2 | Bio-Rad |
| Plasmids | ||
| pCR2.1 | T/A vector for cloning PCR-amplified DNA; Ampr Kanr | Invitrogen |
| pDL289 | E. coli-streptococcal shuttle vector; Kanr | 8 |
| pUC18 | E. coli cloning vector; Ampr | Invitrogen (46) |
| pDC9 | pUC18-based plasmid encoding P1ΔP | 5 |
| pDC9-7 | First P region repeat from spaP cloned into pDC9; Ampr | This study |
| pDC9-15 | First and second P region repeats cloned into pDC9; Ampr | This study |
| pDC9-22 | Complete P region cloned into pDC9; Ampr | This study |
| pDC20 | pUC18-based plasmid harboring full-length spaP; Ampr | 5 |
| pMAJJ8 | pDL289-based plasmid encoding P1ΔP P; Kanr | 5 |
| pTS21 | pDL289-based plasmid encoding P1ΔA; Kanr | 60 |
| pMAD | pDL289-based plasmid harboring full-length spaP; Kanr | 5 |
| pPC967 | Complete P region cloned into pMAJJ8: Kanr | This study |
Anti-P1 MAbs and polyclonal antibodies.
Anti-P1 immunoglobulin G1 (IgG1) MAbs were generated previously (3). Murine ascites fluids served as the sources of the MAbs. Rabbit antiserum 209 was raised against P1 purified from S. mutans strain NG8, and rabbit antiserum 218 was raised against S. mutans NG8 whole cells (5) and rendered monospecific for P1 by exhaustive adsorption with the spaP-negative isogenic mutant PC3370 (10).
Construction of the htrA-spaP double mutants.
Chromosomal DNA from S. mutans strain SAB2, in which the htrA gene had been replaced with an erythromycin cassette (1), was prepared as described previously (17) and used to naturally transform strains PC3370A, -B, -C, and -D (Table 1) by the method described previously (52) to generate PC3370A-ΔhtrA. The double-mutant strains were selected on agar containing kanamycin and erythromycin and confirmed by PCR.
Reintroduction of one, two, or three proline-rich repeats into P1ΔP and Western blot analysis.
Subclones encoding one, two, or all three of the proline-rich tandem-repeat sequences of P1 were constructed based on the known sequence of spaP (28) by PCR amplification using the primers indicated in the table in the supplemental material, followed by ligation to pCR2.1 (Invitrogen) and transformation of E. coli Top10 chemically competent cells (Invitrogen). Insert DNA was excised with ClaI; ligated to the similarly restricted plasmid pDC9 to generate pDC9-7, pDC9-15, and pDC9-22, respectively; and used to transform E. coli MC1061. pDC9 encodes P1ΔP and is a pUC18-based in-frame internal-deletion construct that was generated by PCR amplification of spaP DNA upstream and downstream of the P region and ligation via ClaI restriction sites engineered into the PCR primers (5). Thus, an engineered ClaI replaces the deleted DNA encoding the P region. All PCR-amplified and cloned inserts were confirmed by sequencing at the University of Florida DNA sequencing core facility. For subsequent dot blot analysis in S. mutans, spaP insert DNA from pDC9-22 was restricted and ligated to similarly digested E. coli-streptococcal shuttle vector pDL289 (8) and used to transform the spaP-negative isogenic mutant S. mutans PC3370 (10), via natural transformation (52), to create strain PC967.
E. coli cells harboring each of the spaP-derived clones were lysed by boiling whole cells harvested from 10 ml of culture in 250 μl sodium dodecyl sulfate (SDS) sample buffer for 5 min. Cell debris was removed by centrifugation, and 40 μl of each extract was separated through a 7.5% (wt/vol) SDS-polyacrylamide gel and electroblotted onto nitrocellulose for Western immunoblot analysis with anti-P1 MAbs as described previously (5). E. coli harboring the pUC18 vector only or pDC20 containing unaltered spaP served as negative and positive controls, respectively.
Binding stoichiometry of the P1 A and P regions by continuous variation.
A variation on the Job Plot (22) was used to measure the binding stoichiometry of the A and P regions of P1 required for the formation of the MAb 4-10A epitope. DNA encoding the A or P region was amplified by PCR using the primers listed in the table in the supplemental material, ligated to pGEX-4T-2 (GE Healthcare), and used to transform E. coli strain DH5α for expression as fusion polypeptides with glutathione S-transferase (GST). P1-GST fusion proteins were expressed from isopropyl-β-d-thiogalactopyranoside-induced recombinant E. coli. Cells were lysed by sonication on ice five times for 15 s each time at a power setting of 3 using a Sonic 300 Dismembrator (ARTEK Systems Corporation, Farmingdale, NY). Triton X-100 (1% [vol/vol]) was added and incubated for 30 min at 25°C. The sonicates were centrifuged for 10 min at 12,000 × g, and the supernatants were applied to glutathione-Sepharose 4B affinity resin (GE Healthcare). Bound fusion proteins were eluted with 10 mM reduced glutathione in 50 mM Tris-HCl, pH 8; confirmed by Western immunoblotting using anti-GST rabbit polyclonal antisera (GE Healthcare); and quantified using the bicinchoninic acid protein assay (Sigma-Aldrich).
Purified A region-GST and P region-GST fusion proteins were diluted in 0.1 M sodium carbonate-bicarbonate buffer, pH 9.6, and mixed in seven different molar ratios while a constant total concentration of 0.67 μM was maintained. The mixtures were incubated at 4°C for 1 h, and 100 μl (total, 3.3 pmol) per well was applied in triplicate to a Costar High Binding plate (Corning Inc., Corning, NY), and the plate was incubated for an additional 16 h at 4°C. The coating buffer and unbound Ags were removed, and unreacted sites were blocked with phosphate-buffered saline (PBS) containing 0.3% (vol/vol) Tween 20 (PBS-Tw) for 16 h at 4°C. After the plate was washed, MAb 4-10A was added (1:1,000) and incubated for 2 h at 37°C. Peroxidase-labeled goat anti-mouse IgG (MP Biomedicals) secondary antibody (1:2,000) was used to trace 4-10A binding. The plates were developed with o-phenylenediamine dihydrochloride substrate solution, and the absorbance at 450 nm was recorded using an MPM Titertek model 550 enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Rad, Hercules, CA). Background controls included MAb 4-10A in the absence of fusion proteins and conjugated secondary antibody alone.
Evaluation of restoration of P1 epitopes by competition ELISA.
To determine whether the isolated A and P regions interact in fluid phase to reconstitute the complete epitope recognized by 4-10A, ELISA plate wells were coated with 100 ng of full-length P1. The coating buffer and unbound Ags were removed, and unreacted sites were blocked with PBS-Tw. Full-length P1, purified A region-GST and P region-GST, a 1:1 molar ratio of A region-GST and P region-GST combined, and GST alone were individually mixed, at a final concentration of 1 nM, with anti-P1 MAb 4-10A (1:8,000) and incubated at 4°C for 30 min, and 100 μl of each mixture was applied to the coated wells. The plates were incubated for an additional 2 h at 37°C and washed with PBS-Tw, and the binding of MAb 4-10A to the immobilized P1 was detected as described above. The assay was performed in triplicate, and the percent inhibition of MAb 4-10A binding to immobilized P1 was calculated as follows: 100 − [(mean optical density {OD} of MAb 4-10A + P1-GST fusion/mean OD of MAb 4-10A alone) × 100].
To evaluate whether the native structure of P1 on the S. mutans cell surface requires the presence of sequences upstream and downstream of the A and P regions, E. coli cell lysates containing the recombinant polypeptides CK1 (amino acids [aa] 185 to 963), CK2 (aa 84 to 963), and RR2 (aa 84 to 1218) were prepared as described previously (41), and a competition ELISA was performed in triplicate essentially as described above except that the ELISA plate wells were coated with S. mutans NG8 whole cells (∼105 CFU/well) and 100 μl of threefold serial dilutions in PBS of each polypeptide were incubated in the wells with 100 μl of the conformation-dependent MAb 3-10E (1:1,000).
Detection of P1 by dot blot analysis.
Dot blot analyses were performed using Protran BA85 nitrocellulose membranes (Whatman GmbH, Dassel, Germany) in a 96-well manifold (MiniFold I; Whatman) as described previously (5, 60) with the following modifications. To test for surface-localized P1 on PC967 whole cells, the membrane was probed with anti-P1 MAb 4-10A (1:500). PC3370A and PC3370C served as the negative and positive controls, respectively. As a control for loading of comparable quantities of cells or extracts from the various strains, replicate membranes were routinely reacted with a polyclonal antiserum against anti-S. mutans whole cells (not shown).
To evaluate P1 surface expression in UA159 compared to TW90 (ΔropA) (71) and SM12 (forced-down dnaK expression) (35), cells were grown for 16 h at 37°C in THYE broth and then diluted 1:50 in triplicate tubes of THYE broth, grown at 37°C to a Klett (Klett-Summerson; Klett Mfg. Co., New York, NY) reading of 50, and diluted 1:50 again in THYE broth. The cells were grown to Klett readings of 20 (early exponential phase) or 150 (stationary phase), harvested by centrifugation, and washed twice with PBS. Early-exponential- and stationary-phase cells were suspended in 50% (vol/vol) of the original culture volume of PBS. Twofold serial dilutions of the cell suspensions were made in PBS, and 100 μl of each dilution was applied to nitrocellulose membranes. Anti-P1 MAbs 3-8D, 4-10A, 5-5D, 6-11A, 4-9D, and 1-6F (3, 6, 41) and polyclonal rabbit antisera 209 and 218 were used to probe replicate membranes and developed as described above. Quantification of P1 surface expression was performed by densitometry using the Alpha Innotech Fluorchem imager and software (San Leandro, CA).
For detection of P1 and its derivatives in PC3370A-ΔhtrA, cellular fractions from each double mutant were prepared as follows. Cells cultured overnight in Terleckyj's defined medium (65) were centrifuged, the culture supernatants were filter sterilized through a 0.2-μm filter, and the harvested cells were washed twice in PBS and suspended in PBS to one-fifth their original culture volume. Cytoplasmic contents were also collected from pelleted cells using a 20-ml aliquot of the same overnight culture. These cells were washed twice with PBS and subjected to glass bead breakage three times for 30 s each time in the Mini-Bead Beater 8 apparatus (BioSpec Products, Inc., Bartlesville, OK). The beads were allowed to settle, and residual cells and debris were removed by centrifugation at 16,000 × g for 15 min at 4°C, followed by filter sterilization. For dot blot analysis, 1 ml of culture supernatants, 50 μl of 1:40 dilutions of the whole-cell suspensions, and 50 μl of the Bead Beater preparations were applied in duplicate to the nitrocellulose membrane, which was blocked with PBS-Tw, reacted with rabbit antiserum 218 monospecific for P1, developed, and analyzed as described above.
Immunoelectron microscopy.
Mid-exponential-phase S. mutans cells were fixed in 4% (wt/vol) paraformaldehyde plus 0.5% (wt/vol) glutaraldehyde in 0.1 M phosphate buffer, pH 7.3, for 1 h at 4°C and then enrobed in 2% (wt/vol) Noble agar (Difco Laboratories, Detroit, MI) at 58 to 60°C and processed for transmission electron microscopy according to the method of Dawes (12) using L. R. White resin (Polysciences, Inc., Warrington, PA) for embedding. Thin sections of approximately 60 nm obtained using a Reichert Ultracut S ultramicrotome (Leica, Inc., Deerfield, IL) were placed on carbon-coated nickel grids (Electron Microscopy Sciences, Fort Washington, PA) and reacted with anti-P1 MAb 5-5D, 3-8D, or 6-8C (3), followed by goat anti-mouse IgG (heavy and light chains) (Ted Pella, Inc., Redding, CA) conjugated to 10-nm gold particles (BBI International, Cardiff, United Kingdom) (63). The sections were poststained with 5% (wt/vol) methanolic uranyl acetate and Reynold's lead citrate (56), and the grids were viewed using a JEOL JEM-1210 transmission electron microscope (JEOL USA, Inc., Peabody, MA) at a 120-KeV accelerating voltage. Images of the bacteria were captured using a Kodak Megaplus digital camera (Advanced Microscopy Techniques, Danvars, MA) equipped with Image Pro Plus (MediaCybernetics, Inc., Silver Spring, MD) to acquire the images at various magnifications. Lack of reactivity of the gold-labeled secondary reagent with the negative control strain PC3370A and the test strain PC3370C was confirmed visually. Sample preparation and microscopy were performed at the Electron Microscopy Laboratory, Department of Pathology, College of Veterinary Medicine, University of Georgia, Athens, GA. Fifty sections each of the PC3370A and PC3370C strains were evaluated by counting the gold particles associated with each section following incubation with the anti-P1 A region-P region-dependent MAb 5-5D. Statistical significance was evaluated using Student's t test. As positive controls for the expression of full-length P1 in PC3370C, 50 thin sections each of PC3370A and PC3370C were also compared using anti-P1 MAbs 3-8D (P < 0.000001) and 6-8C (P < 0.01), which map to the N and C termini of P1, respectively, and do not require an A region-P region interaction (5, 6, 11, 60).
Evaluation of S. mutans interaction with immobilized and fluid phase salivary agglutinin.
Adherence of S. mutans whole cells to human salivary agglutinin was assayed by surface plasmon resonance using the BIAcore 3000 (BIAcore AB, Uppsala, Sweden) as previously described (50). Briefly, human salivary agglutinin was immobilized on an F1 sensor chip surface by amine coupling. Suspensions in adherence buffer of S. mutans UA159, TW90 (ΔropA), or AH3370 (ΔspaP) were injected onto the sensor chip, and the change in resonance units was monitored over time.
Aggregation of S. mutans in the presence of fluid phase agglutinin was measured in a spectrophotometer as described previously (7). Briefly, S. mutans cells were suspended in PBS to a Klett value of 250 (corresponding to an OD at 700 nm [OD700] of approximately 1.0). Four hundred microliters of bacterial suspension, 100 μl of PBS, and 6 μl of 0.1 M CaCl2 were mixed in a test tube, vortexed, and transferred to cuvettes (1-cm light path). The cuvettes were equilibrated for 5 min at 37°C in a Shimadzu UV 160 spectrophotometer equipped with a temperature-controlled multicuvette positioner, the samples were automatically positioned, and the absorbance at OD700 was read at 5-min intervals for 1 h. Percent aggregation (the percent decrease in the OD700) was calculated as follows: [(OD700 at 0 min − OD700 at 60 min)/OD700 at 0 min] × 100.
Purification of P1 from wild-type S. mutans and ΔropA mutant strains and evaluation of interaction with immobilized and fluid phase salivary agglutinin.
Proteins from 16-h spent culture supernatants of UA159 and TW90 (ΔropA) were precipitated with ammonium sulfate, dialyzed in PBS, and separated through the Bio-Silect SEC 250-5 size exclusion column (Bio-Rad) using the Biologic DuoFlow HPLC (Bio-Rad). The column was run using 0.5 M Na2HPO4, 0.5 M NaH2PO4, 0.15 M NaCl, 0.02% (wt/vol) NaN3, pH 6.8, as the running buffer at a flow rate of 0.2 ml min−1. Fractions containing a single P1 band were pooled following identification by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis with monospecific rabbit polyclonal anti-P1 antiserum 218. Binding of the purified P1s to immobilized salivary agglutinin was assessed by a BIAcore surface plasmon resonance assay as described above. Samples were diluted in adherence buffer, 10 μl was injected onto the coated chip, and the change in resonance units was recorded over time. To assess the interaction of P1 derived from UA159 compared to TW90 in interacting with fluid phase salivary agglutinin, the abilities of the purified proteins to competitively inhibit the aggregation of S. mutans whole cells were determined using a modification of the assay described above. Each purified protein (2.5 or 5.0 pmol) was added to the bacterial suspension (with the volume adjusted accordingly) for comparison with samples in the absence of inhibitor. The percent inhibition of aggregation was calculated as follows: (percent aggregation without P1 − percent aggregation with P1)/percent aggregation without P1] × 100. Assays were performed in triplicate. The statistical significance was assessed using Student's t test.
Real-time reverse transcription-PCR quantitation of dnaK mRNA.
Cultures of PC3370A to -D were grown in triplicate to Klett 100 (mid-exponential phase). RNA from each culture was isolated according to the supplier's instructions using the Qiagen RNeasy kit (Qiagen, Valencia, CA). Total RNA concentrations were measured by their absorbances at 260 nm. cDNAs of dnaK and 16S RNA were synthesized from 0.5 μg of RNA using primers dnaKAS and 16sRVS (see the table in the supplemental material) and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) for 10 min at 25°C, 50 min at 42°C, and 15 min at 70°C. Transcript levels were determined following PCR with dnaK and the 16S primers listed in the table in the supplemental material with iQ Sybr green Supermix (Bio-Rad, Hercules, CA) in a 25-μl volume using the manufacturer's protocols. Amplification was performed under the following conditions: 30 seconds at 95°C, followed by 40 cycles of 10 seconds at 95°C and 45 seconds at 60°C. Melting-curve data were collected with an additional 100 cycles of 10 seconds starting at 60°C and increasing by 0.4°C after cycle 2 and 15 s at 72°C. DNA amplification and fluorescence detection were performed with the iCycler IQ real-time PCR detection system and the accompanying software (Bio-Rad). A standard curve was plotted for the reaction, with critical threshold values obtained from the amplification of known quantities of DNA from dnaK. Each amplification reaction was performed in triplicate with <10% variation between replicate samples. The experiment was repeated twice with total RNA prepared on separate occasions. The statistical significance was evaluated using Student's t test. 16S RNA was used to normalize RNA abundances for all reactions.
RESULTS
Western blot reactivities of anti-P1 MAbs with P1ΔP containing one, two, or three proline-rich tandem repeats.
Previously, an internal in-frame deletion was generated in which spaP DNA encoding P1 upstream and downstream of the P region was amplified by PCR and ligated in frame via a ClaI restriction site engineered into the PCR primers (5). Elimination of this region destroyed or substantially decreased the binding of four anti-P1 MAbs that did not react with a recombinant P1 polypeptide encompassing the P region. It was subsequently shown by additive ELISA that the epitopes recognized by these MAbs were reconstituted by the interaction of discontinuous A region and P region polypeptides containing various degrees of flanking sequence (41, 57). While the number of A and P region repeats within P1 homologs varies among strains and species, no Ag I/II family protein has been identified that completely lacks either of these two domains. A comparison of Ag I/II family proteins expressed in Lactococcus lactis revealed that Pas of Streptococcus intermedius, which possesses only a single A and a single P region repeat, was secreted, retained at the cell surface, and functionally active, as evidenced by its interaction with immobilized and fluid phase gp340 (24).
Given that several P1 epitopes are reconstituted upon interaction of the A and P regions, we first sought to determine the minimal number of P region repeats required for restoration of A region/P region conformational epitopes. spaP DNA encoding one, two, or three P region repeats was amplified by PCR and ligated in frame by virtue of the engineered ClaI site that remained after deletion of P region-encoding DNA. The recombinant polypeptides were expressed in E. coli, and reactivity with a comprehensive panel of anti-P1 polyclonal antibodies and MAbs was determined by Western blotting (Fig. 2). Background reactivity with the vector-only negative control sample varied and is shown for all the antibodies tested (lane a of each panel). Lanes b through f show the samples containing P1ΔP; P1ΔP reconstituted with one, two, or three proline-rich repeats; and full-length P1, respectively. Similar levels of reactivity against all five P1 polypeptides were observed using two different anti-P1 polyclonal antisera, 218 and 209. Both reagents were employed because they were raised against different immunogens. Antiserum 218, raised against S. mutans whole cells, recognizes the isolated P region, while antiserum 209, raised against purified soluble P1, does not (5). Changes in immunoreactivity among the P1 variants were not readily discernible with either of these polyclonal reagents. Together, these results indicate comparable levels of expression of each of the recombinant molecules, which was also confirmed by protein staining (not shown).
FIG. 2.
Western immunoblot analysis of P1ΔP reconstituted with P region repeats. Recombinant P1 polypeptides were separated on replicate 10% SDS-polyacrylamide gels, and the corresponding nitrocellulose membranes were reacted with the indicated anti-P1 polyclonal and monoclonal reagents. Lanes a through f contain samples derived from E. coli harboring the pUC18 vector-only control and plasmids pDC9 (P1ΔP), pDC9-7 (one repeat), pDC9-15 (two repeats), pDC9-22 (three repeats), and pDC20 (containing unaltered spaP), respectively. The migration of molecular mass standards is indicated on the left of the upper panels, and the apparent molecular masses of the full-length P1 and P1ΔP polypeptides are indicated on the right.
Although the predicted molecular mass of full-length P1 after cleavage of the signal sequence is ∼167 kDa, the protein migrates on SDS-polyacrylamide gels at ∼185 kDa. Elimination of either the P region or the A region causes P1 to migrate close to its predicted molecular mass, suggesting that an SDS-resistant intramolecular interaction may contribute to the observed discrepancy (5, 60). The predicted molecular masses of P1ΔP and P1ΔP reconstituted with one, two, and three proline-rich repeats are ∼148, ∼155, ∼159, and ∼167 kDa, respectively. Interestingly, all of these polypeptides, including P1ΔP reconstituted with the complete P region, migrated close to the predicted mass rather than the aberrant migration that is characteristically observed for full-length P1. As a consequence of the introduction of the ClaI site during the initial engineering of the P region deletion, reintroduction of deleted DNA resulted in residual ClaI sites on either side of the restored sequence. Therefore, two additional amino acids, isoleucine and aspartic acid, now flanked the P region in the reconstituted polypeptide. Because of the notable effect the introduction of only 4 amino acids had on P1's migration by SDS-PAGE, all of the constructs were carefully confirmed by sequence analysis. Apparently, this seemingly minor change in sequence was sufficient to impede the SDS-resistant intramolecular interaction, which likely caused P1 to migrate more slowly than predicted.
When the reactivities of the anti-P1 MAbs were evaluated, the results varied and in many cases were dependent on the number of reintroduced P region repeats. MAbs 6-8C, 2-8G, 3-3B, and 5-3E map to the C terminus of P1. Their binding is not destroyed by elimination of either the A or P region (5, 6, 41, 60). This group of MAbs reacted with all five polypeptides, and increasing the number of repeats appeared to improve their binding, suggesting a potential effect on epitope exposure. Reintroduction of a single repeat fully restored the binding of the A region/P region-dependent MAb 4-10A. This indicates that the additional isoleucine and aspartic acid residues now flanking the P region did not prevent the A region-P region interaction necessary to generate this epitope. MAbs 1-6F and 4-9D do not depend on an A region-P region interaction and map to the segment of P1 intervening between the two domains (41). Their binding to the reconstituted polypeptides (Fig. 2, lanes c to e) was increased compared to the unaltered molecule (lanes f), indicating that their epitopes became better exposed upon reintroduction of the P region, likely due to the structural alteration resulting from the introduction of the four additional amino acids. This is consistent with previous results that indicated that certain epitopes are masked or cryptic in the context of the complete unaltered sequence (41). MAbs 5-5D and 6-11A share the common properties of having their core epitopes reconstituted by an interaction of A and P region polypeptides, but unlike 4-10A, their binding as assessed by additive ELISA was enhanced by inclusion of sequence immediately upstream of the A region (41, 57). These two MAbs again were similar to one another, and their binding improved as the number of reintroduced proline-rich repeats was increased. This reiterates that the formation of certain conformational epitopes is facilitated by the presence of more complete P region sequence.
Lastly, binding of MAb 3-10E is destroyed by elimination of the P region and decreased by elimination of the A region (60), but its epitope is not restored by a simple interaction of these two domains (41). Its binding is also destroyed by elimination of the amino acid residues 84 to 190 immediately upstream of the A region, and its epitope is reconstituted only upon interaction of A and P region-containing polypeptides that also include pre-A region and post-P region sequence, respectively. Collectively, these data suggest that within P1 the A region-P region interaction facilitates a pre-A region-post-P region interaction that then achieves the conformational epitope recognized by 3-10E. Binding of MAb 3-10E was not restored by the reintroduction of one, two, or three proline-rich repeats (Fig. 2, lanes c to e) into P1ΔP (lane b). The restoration of binding of MAb 4-10A indicated that an A region-P region interaction was achieved in all of these constructs; therefore, the presence of the isoleucine and aspartic acid residues on either side of the reintroduced proline-rich repeats appears to prevent the pre-A region-post-P region interaction necessary for the formation of the 3-10E epitope. It also suggests that it is the pre-A region-post-P region interaction that is largely responsible for the retarded migration of P1 on SDS-polyacrylamide gels.
While the notable alteration in P1's structure resulting from the introduction of four additional amino acids upon reintroduction of the deleted sequence was unexpected and inadvertent and was due to the initial strategy used to engineer P1 devoid of the P region, it was fortuitous in that it afforded insight into additional structural features of P1. As described below, this allowed us to assess the requirement for an A region-P region interaction in the surface localization of P1 independent of other intramolecular interactions.
Further evaluation of P1 interactions recognized by conformation-dependent MAbs.
We wished to confirm that the complete epitope recognized by MAb 4-10A is fully reconstituted by interaction of the isolated A and P region domains out of the context of the rest of the molecule. A competition ELISA was used for this purpose (Fig. 3A). A mixture of A region- and P region-GST fusion polypeptides was as effective as full-length P1 at inhibiting the binding of MAb 4-10A to immobilized P1. No inhibition was detected in the presence of either polypeptide alone or the GST-only negative control. These results firmly established that the epitope recognized by 4-10A is solely dependent on an interaction of the A and P region domains, and it was therefore used in the subsequent continuous-variation assay to determine the stoichiometry of A and P region binding. Various molar ratios of A region- and P region-GST fusion proteins were mixed while a constant total molar concentration was maintained (Fig. 3B), and the optimal interaction between the A and P regions was clearly seen at a 1:1 molar ratio.
FIG. 3.
Evaluation of discontinuous epitopes recognized by anti-P1 MAbs 4-10A and 3-10E. (A) Reconstitution of the MAb 4-10A epitope. Inhibition of MAb 4-10A binding to immobilized P1 was measured by competition ELISA following incubation with the indicated A region- and P region-GST fusion proteins or with full-length P1 and GST-only positive and negative controls. Percent inhibition was calculated as follows: 100 − [(mean OD450 of MAb 4-10A + P1 or GST fusion{s}/mean OD450 of MAb 4-10A alone) × 100]. (B) Stoichiometry of the A region-P region interaction by continuous-variation analysis. Inverse amounts of A and P region-GST fusion proteins (total, 3.3 pmol/well) were incubated together, and ELISA plate wells were coated with them. Epitope formation was traced with MAb 4-10A. The molar percentages of the A and P region polypetides are indicated on the x axis. (C) P1 sequence requirements for achievement of the MAb 3-10E epitope on the surface of S. mutans. Inhibition of MAb 3-10E binding to S. mutans whole cells was measured by competition ELISA following incubation with threefold serial dilutions of E. coli lysates containing RR2 (filled diamonds), CK1 (filled triangles), CK2 (open circles), or the vector-only controls (× and filled squares). The percent inhibition was calculated as follows: 100 − [(mean OD450 of MAb 3-10E + P1 polypeptide/mean OD450 of MAb 3-10E alone) × 100]. Error bars indicate standard deviations.
To confirm that the simultaneous presence of sequence upstream and downstream of the A and P regions in fact contributes to the native structure of P1 as it exists on the S. mutans cell surface, a competition ELISA using MAb 3-10E was performed (Fig. 3C). Only recombinant polypeptide RR2 (aa 84 to 1218), which includes both pre-A and post-P region sequence, was able to compete for binding of MAb 3-10E to whole bacterial cells. There was no inhibition observed in the presence of the vector-only negative control. Neither polypeptide CK1 (185 to 963) nor CK2 (84 to 963), which includes pre-A region but not post-P region sequence, was able to achieve the structure necessary to inhibit the binding of 3-10E. Of note, the migration of RR2 as detected by SDS-PAGE was also retarded by ∼18 kDa, while both the CK1 and CK2 polypeptides migrated according to their predicted molecular masses (41).
Restoration of P1 surface expression by reintroduction of P region sequence within P1ΔP.
To determine whether the restoration of the A region-P region interaction is sufficient to rescue surface expression of P1 on S. mutans, whole cells of PC967 (P1ΔP reconstituted with three proline-rich repeats), as well as the control strain PC3370A (vector only) or PC3370C (expressing unaltered P1), were diluted serially, applied to nitrocellulose, and reacted with MAb 4-10A (Fig. 4A). The results demonstrated that upon reintroduction of the P region into P1ΔP, the A region-P region interaction was accomplished and P1 was once again detectable on the cell surface of S. mutans. To ensure that the 4-10A MAb reactivity demonstrated by dot blotting resulted from translocation of the intact molecule and not fragments that might interact to restore the epitope, Western blot analysis of SDS extracts of whole cells was also performed (Fig. 4B). Streptococci are not lysed by boiling them in detergent; therefore, immunoreactive material is indicative of molecules present on the outside of the cell. A band of the appropriate size was detected in the SDS extract of the rescued S. mutans strain, PC967. While the level of extracellular P1 was less than that detected for the unadulterated P1 molecule, its presence was clearly observable and suggests that restoration of an A region-P region interaction confers secretion competence even in the absence of restoration of completely native structure. Binding of other anti-P1 antibodies, including 3-10E, to the surface of PC967 was not observed (not shown), indicating that the reengineered P1 localized on the cell surface of this strain is not identical in conformation to that of native P1.
FIG. 4.
Restoration of P1 surface expression on S. mutans by reintroduction of the P region into P1ΔP. (A) Whole-cell dot blot analysis. The indicated strains of S. mutans were serially diluted twofold, and the filter was reacted with anti-P1 MAb 4-10A. (B) Western blot analysis. SDS extracts of the indicated strains were separated on 10% SDS-polyacrylamide gels, and the blots were reacted with MAb 4-10A.
Demonstration of an intracellular A region-P region interaction within P1.
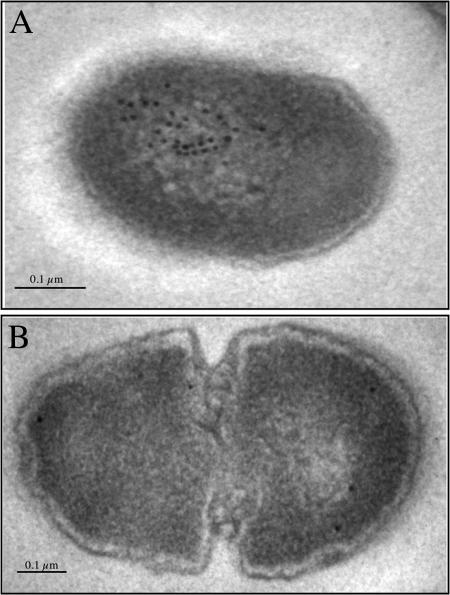
Taken together, the demonstration that elimination of either the A region or the P region of P1 prevents surface localization of the protein, that the discontinuous A and P regions interact to achieve complex epitopes, and that cell surface P1 is observed again upon reintroduction of deleted P region sequence within P1ΔP, strongly suggests that the A region-P region interaction contributes to the proper secretion and translocation of P1. While it seems counterintuitive that a protein would first fold within the cell only to unfold while transiting the translocon pore in an unfolded state, the complexities of the bacterial secretion apparatus, particularly in less studied organisms, such as the streptococci, are far from fully defined. Recently, the ability of a bacterial Sec translocase to actively unfold and translocate tightly folded preproteins was demonstrated in E. coli, the most widely studied bacterial secretion model (47). To determine whether P1 folds within the cell prior to its secretion, we utilized immunoelectron microscopy and employed anti-P1 MAb 5-5D, whose epitope is not achieved unless there is an interaction between discontinuous A and P region-containing sequences (41). Thin sections of PC3370C were incubated with anti-P1 MAb 5-5D and traced with gold-labeled secondary antibody (Fig. 5). PC3370A served as the negative control strain for nonspecific background binding. Fifty sections per strain were counted. An average of 4.06 ± 1.83 particles/section were observed in PC3370C compared to 1.55 ± 1.55 particles/section for PC3370A (P ≤ 0.005). The immunodetection of intracellular P1 by the conformation-dependent MAb 5-5D in PC3370C compared to PC3370A confirmed that an intramolecular interaction within P1 occurs within the cell prior to translocation of the molecule.
FIG. 5.
Demonstration of intracellular folding of P1 by immunoelectron microscopy. Shown is transmission electron microscopy of representative thin sections of S. mutans PC3370C (A) or PC3370A (B) reacted with the A and P region-dependent anti-P1 MAb 5-5D, followed by gold-labeled goat anti-mouse IgG. Bars, 0.1 μm.
RopA (trigger factor) and DnaK are involved in P1 maturation and surface expression.
Given their established roles as chaperones, the next series of experiments were undertaken to evaluate the potential contributions of S. mutans RopA and DnaK to the presence and function of P1 on the cell surface. We utilized two previously engineered mutant strains of S. mutans. TW90 is a ropA-negative mutant strain (71). A complete knockout of dnaK has not been achieved to date and is likely not isolable; however, a knockdown strain, SM12, that expresses <5% of wild-type DnaK levels has been generated (35).
Ag I/II family molecules mediate the Ca2+-dependent binding of the oral streptococci that express them to human salivary agglutinin gp340 glycoprotein. A BIAcore surface plasmon resonance assay was developed previously to assess the interaction of S. mutans whole cells with immobilized agglutinin and to evaluate the abilities of antibodies to disrupt that binding (23, 50). In this system, measured adherence has been shown to be P1 mediated by comparing wild-type S. mutans strain NG8 with the spaP-negative isogenic mutant PC3370. When this assay was used to determine the effect of elimination of RopA on the adherence of S. mutans, a substantial reduction was observed for TW90 compared to its wild-type parent strain, UA159 (Fig. 6A). Minimal binding was detected for the negative control AH3370 (ΔspaP) generated in the UA159 background. The inhibition of adherence of both UA159 and TW90 by MAb 4-10A (not shown) confirmed that the residual adherence detected for TW90 is P1 mediated.
FIG. 6.
Effects of deletion of ropA on the interaction of S. mutans P1 with immobilized and fluid phase human salivary agglutinin. (A) Adherence of S. mutans whole cells. BIAcore surface plasmon resonance analysis was used to evaluate the binding of S. mutans UA159, TW90 (ΔropA), and AH3370 (ΔspaP) whole cells to the agglutinin-coated chip. The change in resonance units (ΔRU) over time is indicated. (B) Aggregation of S. mutans whole cells. A spectrophotometric assay, as indicated by a decrease in OD600 over time, was used to monitor the aggregation of the indicated strains in the presence of fluid phase agglutinin. The error bars indicate standard deviations. (C) Visualization of P1 purified from ΔropA and wild-type strains of S. mutans. Shown are colloidal-gold protein stain (left three lanes) and Western blot analysis (right two lanes) of P1 purified from TW90 compared to UA159. Monospecific anti-P1 rabbit polyclonal antiserum 218 was used for the Western blot. Lane M, molecular mass standards. (D) Interactions of P1 purified from wild-type and ΔropA backgrounds with immobilized agglutinin. The interaction of equivalent amounts (5 pmol) of P1 purified from TW90 compared to UA159 was assessed by BIAcore assay. Identical results were obtained with 2.5 pmol of protein (not shown).
Whole-cell dot blot and densitometry analyses were used to determine whether the reduction in adherence of TW90 was due to a reduced level of surface-localized P1. No significant difference was detected between wild-type UA159 and TW90 when nitrocellulose membranes were reacted with each of five different anti-P1 MAbs known to react with cell surface P1, as well as with the two rabbit polyclonal antisera. Thus, RopA does not appear to affect the amount of cell surface-localized P1 but does appear to contribute to a modification of P1 to a fully functional form. This modification does not result in a gross change to the molecule, since the antigenicity of P1, as recognized by currently available monoclonal and polyclonal reagents, was unaltered.
To determine whether the RopA-dependent modification of P1 affected its ability to interact with fluid phase salivary agglutinin, an aggregation assay was also performed (Fig. 6B). There was no difference in the aggregation properties of UA159 and TW90 when bacterial suspensions were mixed with fluid phase agglutinin in the presence of calcium and the OD was monitored over time. By comparison, AH3370 did not aggregate at all in the presence of fluid phase agglutinin, confirming that the measured S. mutans-agglutinin interaction reflects the presence of P1. Taken together, these results indicate that RopA's effect on P1 is targeted to the adherence-promoting function and does not influence the overall level of cell surface P1 or its ability to interact with the fluid phase form of agglutinin.
To determine whether RopA mediates a direct or downstream modification of P1 itself, rather than the alteration in adherence stemming primarily from a modification of other molecules that could potentially alter P1's adhesive function in the context of the bacterial surface, P1 was purified from UA159 and TW90. A colloidal-gold protein stain (left two lanes) and Western blot (right two lanes) of P1 from each strain are shown in Fig. 6C. When 500 nM (not shown) or 1,000 nM concentrations of protein from UA159 or TW90 were reacted with agglutinin immobilized on the sensor chip surface, there was a substantial reduction in the binding of purified P1 derived from the RopA-deficient mutant strain (Fig. 6D). However, when these same protein preparations were tested for the ability to inhibit P1-mediated aggregation of wild-type S. mutans in the presence of fluid phase agglutinin, no significant difference was detected. The percent inhibition of aggregation for UA159-derived P1 compared to TW90-derived P1 was 32.1% ± 3.2% versus 37.8% ± 7.2% (P = 0.2) when 50 nmol of each purified protein was added to the S. mutans bacterial suspension. The values were 60.2% ± 15.4% and 60.5% ± 10.9%, respectively (P = 0.98), when 200 nmol of purified proteins was used. Hence, the absence of RopA results in a functional alteration of P1 itself that diminishes the ability of S. mutans to interact with immobilized, but not fluid-phase, agglutinin.
To examine the contribution of DnaK to P1 surface expression, a dot blot experiment similar to that described above for TW90 was performed using the S. mutans dnaK knockdown strain SM12 (35). At early exponential phase, there was a significant reduction in the detection of surface-localized P1 associated with SM12 (2,500 ± 150 OD units) compared to UA159 (3,500 ± 200 OD units) (P < 0.001), suggesting the involvement of this chaperone in P1 translocation. However, by stationary phase, P1 surface expression in SM12 was found to be equivalent to that of the wild-type strain. Thus, the transport of P1 to the cell surface was delayed but not prevented upon DnaK down-regulation, and the low-level residual expression of this chaperone in SM12 may be sufficient to enable P1 to traffic properly.
The lack of a completely isogenic knockout mutant limits the strategy of using a mutant background to establish more firmly a role for DnaK in the surface expression of functional P1. However, given its multifactorial and extensive role as a chaperone and its known contribution to the degradation and turnover of malfolded proteins within the cell, we tested whether dnaK message levels were altered in response to the expression of P1 or either of the internal in-frame deletion derivatives. Quantitative real-time PCR was used to measure the DnaK message copy numbers in PC3370A (9.99 × 104), PC3370B (9.03 × 103), PC3370C (2.40 × 104), and PC3370D (6.52 × 105). In the presence of P1ΔA, the level of dnaK message was increased compared to the vector-only (P < 0.005) or full-length P1 (P < 0.05) control. In the presence of P1ΔP, the level of dnaK message was decreased compared to the vector-only (P < 0.005) or full-length (P < 0.05) P1. There was no significant difference in dnaK message levels between the vector-only and full-length P1 strains.
P1ΔP accumulates within the cell in the absence of HtrA.
The chaperone-protease HtrA/DegP has been shown to contribute in large part to protein quality control in the periplasmic spaces of gram-negative bacteria (51). The streptococci possess a single HtrA homolog. Bacterial HtrA has a known housekeeping function in the turnover of damaged or malfolded proteins that may accumulate, particularly under stress conditions, such as high temperature, where HtrA functions predominantly as a protease (62). It also serves as an extracellular protease in the processing and maturation of proproteins and the degradation of abnormal exported proteins in L. lactis (53). Therefore, we wished to determine whether HtrA affects the translocation and/or turnover of P1 or its internal-deletion derivatives. We took advantage of a mutant strain of S. mutans UA159, SAB2, in which htrA had been completely replaced with an erythromycin resistance gene (1), and superimposed that mutation into the PC3370A to -D strains by transformation with chromosomal DNA from the ΔhtrA mutant. Dot blot analysis of whole bacteria, culture supernatants, and cytoplasmic contents from the parental and corresponding htrA knockout strains is shown in Fig. 7. There was an increase in the detection of P1ΔP in the cytoplasmic fractions of cells lacking HtrA (4,108 ± 18 compared to 690 ± 62 densitometry units); however, the opposite effect was observed in the cyoplasmic fractions of PC3370C, where more P1 was detectable in the presence (1,588 ± 363 densitometry units) than in the absence (394 ± 20 densitometry units) of HtrA. These results are consistent with the multifunctional nature of HtrA and its ability to switch from a chaperone to a protease depending on the prevailing environment. Deletion of htrA did not result in the detection of P1ΔP or P1ΔA on the cell surface, nor was there an accumulation of these polypeptides in the culture supernatants. These results suggest that HtrA does play a role in the intracellular fate of P1. The cytoplasmic accumulation of P1ΔP in the htrA-negative background points to a contribution of an HtrA protease function in the degradation of this altered form of the molecule and strengthens the speculation that malfolded P1 is unstable and is degraded intracellularly without being secreted.
FIG. 7.
Dot blot analysis of the localization of P1 and its internal-deletion derivatives in htrA-positive and htrA-negative strains of S. mutans. P1 and the internal-deletion polypeptides present in the indicated fractions of PC3370A to -D and corresponding ΔhtrA strains were identified by dot blotting using monospecific anti-P1 rabbit polyclonal antiserum 218.
DISCUSSION
This study examined factors contributing to the successful surface localization and function of the adhesin P1 of S. mutans, including structural aspects of the molecule itself, as well as chaperones and a protease known to be involved in the maturation and turnover of other proteins from gram-positive organisms. We used the A region-P region-dependent anti-P1 MAb 4-10A to demonstrate that interaction of these two discontinuous domains, even without restoration of full native structure, is sufficient to confer surface localization of the protein. The translocated P1 represents a single polypeptide of predicted size and is not simply a composite of protein fragments that together might regenerate the epitope. In addition, we used immunoelectron microscopy and the anti-P1 MAb 5-5D, an antibody that also depends on an interaction between the A and P regions to achieve its cognate epitope, to establish that P1 at least partially folds within the cell prior to its secretion. Taken together, these results suggest not only that intracellular folding of P1 occurs, but that an A region-P region interaction is a requisite element for its proper translocation.
Homology to the P region of P1 is found in several surface proteins of both prokaryotes and eukaryotes. Among those in gram-positive organisms are the fibronectin binding proteins of Streptococcus pyogenes (64) and Staphylococcus aureus (61), immunogenic secreted protein (Isp) of S. pyogenes (42), and the virulence-associated surface protein PspA of Streptococcus pneumoniae (72). Proline-rich regions are known to be involved in a variety of intra- and intermolecular protein-protein interactions, including chaperone-like activities. In Limulus polyphemus (the horseshoe crab), the modular multidomain 132-kDa secreted serine protease factor C contains a centrally located proline-rich region that is necessary for the folding, stability, and secretion of the protein (70). Because the effect was similar to that previously described by us for the P region of P1, the authors speculated that internal proline-rich regions might act as intramolecular chaperones for the correct folding and secretion of proteins that contain them. Ma et al. (38) have also proposed a model of protein folding guided by domain-domain interactions and posited that intramolecular chaperone-like building blocks function within many proteins to influence folding and dynamics, but that unlike intermolecular chaperones, the interaction is not transient and the foldases remain attached and form an integral part of the mature protein. Our data are consistent with an intracellular folding mechanism involving discontinuous domains of P1 that precede its translocation and enable it to interact in an appropriate manner with the bacterial secretion apparatus. Of interest, an extensively shortened P1 polypeptide encoded by p26R3 (33) that corresponds to the amino-terminal 480 aa of P1 and terminates just C terminal to the A region, is secreted into the periplasm of E. coli and into the culture supernatant of S. mutans (not shown), although it is not detectable on the cell surface, as would be expected given the lack of C-terminal wall-anchoring sequence. P1ΔP contains all the primary sequence that the truncated construct has and more, yet it is not externalized. Intermolecular chaperones, such as SecB, lacking in gram-positive organisms, have been shown to hold substrates in a loosely folded secretion-competent state whereby the signal peptide can be recognized by the secretion machinery (55). It is possible that in the context of the full-length protein an intramolecular interaction holds P1 in a conformation so that its N-terminal signal sequence is accessible and not buried within a malfolded polypeptide. The recent identification of an unfoldase activity of SecA, the ATPase motor that drives transiting polypeptides through the SecYEG protein-conducting channel, and the demonstration that even a tightly folded preprotein can be translocated efficiently by the bacterial Sec translocase (47) strengthens our conclusion that intracellular folding of P1 would not preclude, and in fact is necessary for, its proper targeting and secretion.
We also examined the roles of several known accessory molecules in the proper localization and function of P1. The first chaperone or folding catalyst that interacts with nascent presecretory proteins is the peptidyl prolyl isomerase trigger factor (21, 68). In addition to isomerase activity, trigger factor has chaperone activity. It prevents the aggregation of proteins, either alone or in combination with GroEL-GroES (45), and functions in the breakdown of abnormal proteins (27). The PPIase activity of trigger factor is required by some, but not all, of the proteins that require it for proper folding (32). In S. pyogenes, trigger factor (RopA) is essential for both the secretion and maturation of the cysteine protease SpeB. Lyon and Caparon (37) demonstrated that in the absence of RopA, the nascent protease polypeptide was not targeted to the secretory pathway. An in-frame deletion of the PPIase domain within RopA resulted in a secreted but enzymatically inactive protease, suggesting that trigger factor has an additional role in protease maturation. It is interesting that the lack of isomerization can send the protease into an alternative folding pathway that results in a malfunctioning enzyme yet does not appear to effect its secretion. In S. mutans, RopA has been shown to be involved in stress tolerance and biofilm formation (71). In this study, we found that elimination of RopA did not have any apparent affect on the amount of surface-localized P1 but did alter its functional activity. Specifically, P1 was impaired in its ability to interact with immobilized, but not fluid phase, salivary agglutinin. This effect involves a modification of P1 itself because whole bacterial cells, as well as the purified adhesin, both demonstrated a diminished interaction with the immobilized ligand by BIAcore assay. Whether RopA modifies P1 directly, via PPIase activity or otherwise, or modifies another component that subsequently contributes to P1's maturation remains to be seen and will be important to dissect. The functional distinction between P1 fully capable of interacting with fluid phase but not immobilized agglutinin is key from a virulence standpoint, as interaction with the former would promote bacterial aggregation and clearance from the oral cavity and benefit the host while interaction with the latter would promote bacterial adherence to the salivary pellicle on the tooth surface and benefit the pathogen.
DnaK, another multifunctional chaperone and component of the complex protein-folding and targeting machinery, was also evaluated, and the results suggested its participation in P1 turnover and surface localization. Our results point to a delay in P1 translocation when dnaK expression is down-regulated but do not necessarily indicate a direct interaction of DnaK with P1, as the reduced surface expression may represent a global effect on protein translocation in general. Quantitative real-time PCR demonstrated a significant increase in dnaK expression in the presence of P1ΔA compared to full-length P1 but a significant decrease in the presence of P1ΔP. In E. coli, transcription of dnaK is initiated by a σ32 promoter that DnaK is involved in regulating by interacting with the σ32 subunit of RNA polymerase, and it was proposed that the presence of denatured proteins acts as a sink for DnaK, thereby freeing σ32 and initiating dnaK transcription (9). In S. mutans, dnaK is transcribed from a σA-type promoter (25) and is negatively regulated by HrcA (34). It is plausible that in the absence of its intramolecular binding partner the exposed P region in the malfolded P1ΔA polypeptide may act as a sink for DnaK, resulting in dnaK up-regulation in a manner of transcriptional control that is similar to that seen with the E. coli σ32. The interaction of DnaK with proline-rich sequences is well documented, and numerous antibacterial proline-rich oligopeptides suppress the metabolism of bacteria by stereospecific binding to DnaK and inhibition of chaperone-dependent protein folding (39). The reciprocal results observed with P1ΔA and P1ΔP and down-regulation of dnaK in the absence of the proline-rich domain of P1 argue that recognition of this sequence indeed plays a role in the interplay with DnaK and the cellular response to structurally altered variants of P1.
Differential results were also observed when P1 variants were expressed in S. mutans lacking the HtrA chaperone-protease. P1ΔP accumulated in the cytoplasm of the htrA mutant, while higher levels of full-length protein were detected in the presence of HtrA. This suggests that the protease activity of HtrA contributes to the turnover of malfolded P1ΔP and again implies an intracellular sensing mechanism for detection of native and altered P1. S. mutans has a single HtrA homolog that was identified by BLAST search as a homolog of DegP, although it has only a single PDZ domain, which according to the E. coli classification scheme would identify it as DegS like (reviewed in reference 30). Unlike other bacterial quality control proteins, such as ClpXP, ClpAP, and HxlUV (16), HtrA proteins do not contain ATP binding domains. Therefore, where other chaperone-proteases are regulated by ATP hydrolysis, HtrA function is controlled by a temperature-dependent switch mechanism. The switch mechanism is also regulated by substrate recognition. Sufficient biochemical studies of streptococcal HtrA have not yet been performed to enable insight into the molecular mechanisms controlling its function and protease activation. The difference in the relative cytoplasmic levels of P1ΔP and full-length P1 observed in S. mutans containing and devoid of HtrA suggests that its function differs depending on the form of the P1 polypeptide that is encountered. In S. mutans, HtrA appears to be part of a regulatory network that connects cellular growth, stress tolerance, biofilm formation, and competence development (1). In L. lactis, an extracelluar HtrA/DegP family protease was identified that participated in the housekeeping of exported proteins (53); however, HtrA did not appear to contribute to the processing of extracellular proteins of S. mutans (13). We found no evidence for an extracellular effect of HtrA in our system.
In summary, in this study, we increased our understanding of intramolecular interactions within P1 that contribute to epitopes recognized by anti-P1 MAbs. This enabled their use as tools to demonstrate that an A region-P region interaction is necessary and sufficient to confer surface expression of the protein in the absence of achievement of full native structure. At least partial folding of the molecule occurs within the cell prior to secretion, and P1 is secreted but does not achieve full functional activity in the absence of RopA. DnaK and HtrA both appear to contribute to the intracellular fate of the protein, depending on its conformational form. As P1 shares architectural features with a number of streptococcal and staphylococcal surface proteins, this additional information will contribute to a more complete understanding of the complexities of protein translocation, maturation, and quality control in other, related organisms.
Supplementary Material
Acknowledgments
This work was supported by the National Institute for Dental and Craniofacial Research grants DE08007 and DE13882 to L.J.B. and training grant T32-DE07200.
We thank Zezhang Wen, José Lemos, and Sang-Joon Ahn for providing the S. mutans mutant strains and Jacqueline Abranches for technical assistance with the quantitative real-time PCR.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 24 March 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ahn, S. J., J. A. Lemos, and R. A. Burne. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 1873028-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 9914434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayakawa, G. Y., L. W. Boushell, P. J. Crowley, G. W. Erdos, W. P. McArthur, and A. S. Bleiweis. 1987. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect. Immun. 552759-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady, L. J., P. J. Crowley, J. K. Ma, C. Kelly, S. F. Lee, T. Lehner, and A. S. Bleiweis. 1991. Restriction fragment length polymorphisms and sequence variation within the spaP gene of Streptococcus mutans serotype c isolates. Infect. Immun. 591803-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady, L. J., D. G. Cvitkovitch, C. M. Geric, M. N. Addison, J. C. Joyce, P. J. Crowley, and A. S. Bleiweis. 1998. Deletion of the central proline-rich repeat domain results in altered antigenicity and lack of surface expression of the Streptococcus mutans P1 adhesin molecule. Infect. Immun. 664274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady, L. J., D. A. Piacentini, P. J. Crowley, and A. S. Bleiweis. 1991. Identification of monoclonal antibody-binding domains within antigen P1 of Streptococcus mutans and cross-reactivity with related surface antigens of oral streptococci. Infect. Immun. 594425-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady, L. J., D. A. Piacentini, P. J. Crowley, P. C. Oyston, and A. S. Bleiweis. 1992. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 601008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley, N. D., L. N. Lee, and D. J. LeBlanc. 1995. Use of a novel mobilizable vector to inactivate the scrA gene of Streptococcus sobrinus by allelic replacement. J. Bacteriol. 1775028-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukau, B. 1993. Regulation of the Escherichia coli heat-shock response. Mol. Microbiol. 9671-680. [DOI] [PubMed] [Google Scholar]
- 10.Crowley, P. J., L. J. Brady, S. M. Michalek, and A. S. Bleiweis. 1999. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect. Immun. 671201-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley, P. J., L. J. Brady, D. A. Piacentini, and A. S. Bleiweis. 1993. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect. Immun. 611547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawes, C. J. 1971. Biological techniques in electron microscopy. Barnes & Noble, New York, NY.
- 13.Diaz-Torres, M. L., and R. R. Russell. 2001. HtrA protease and processing of extracellular proteins of Streptococcus mutans. FEMS Microbiol. Lett. 20423-28. [DOI] [PubMed] [Google Scholar]
- 14.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 41603-1605. [DOI] [PubMed] [Google Scholar]
- 15.Forester, H., N. Hunter, and K. W. Knox. 1983. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J. Gen. Microbiol. 1292779-2788. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman, S. 2003. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19565-587. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez, J. A., P. J. Crowley, D. P. Brown, J. D. Hillman, P. Youngman, and A. S. Bleiweis. 1996. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J. Bacteriol. 1784166-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajishengallis, G., T. Koga, and M. W. Russell. 1994. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J. Dent. Res. 731493-1502. [DOI] [PubMed] [Google Scholar]
- 19.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasona, A., P. J. Crowley, C. M. Levesque, R. W. Mair, D. G. Cvitkovitch, A. S. Bleiweis, and L. J. Brady. 2005. Streptococcal viability and diminished stress tolerance in mutants lacking the signal recognition particle pathway or YidC2. Proc. Natl. Acad. Sci. USA 10217466-17471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hesterkamp, T., S. Hauser, H. Lutcke, and B. Bukau. 1996. Escherichia coli trigger factor is a prolyl isomerase that associates with nascent polypeptide chains. Proc. Natl. Acad. Sci. USA 934437-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, C. A. 1982. Determination of binding stoichiometry by the continuous variation method: the Job Plot. Enzyme Kinetics Mech. 87509-525. [DOI] [PubMed] [Google Scholar]
- 23.Isoda, R., R. A. Robinette, T. L. Pinder, W. P. McArthur, and L. J. Brady. 2007. Basis of beneficial immunomodulation by monoclonal antibodies against Streptococcus mutans adhesin P1. FEMS Immunol. Med. Microbiol. 51102-111. [DOI] [PubMed] [Google Scholar]
- 24.Jakubovics, N. S., N. Stromberg, C. J. van Dolleweerd, C. G. Kelly, and H. F. Jenkinson. 2005. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol. Microbiol. 551591-1605. [DOI] [PubMed] [Google Scholar]
- 25.Jayaraman, G. C., J. E. Penders, and R. A. Burne. 1997. Transcriptional analysis of the Streptococcus mutans hrcA, grpE and dnaK genes and regulation of expression in response to heat shock and environmental acidification. Mol. Microbiol. 25329-341. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson, H. F., and D. R. Demuth. 1997. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23183-190. [DOI] [PubMed] [Google Scholar]
- 27.Kandror, O., M. Sherman, and A. Goldberg. 1999. Rapid degradation of an abnormal protein in Escherichia coli proceeds through repeated cycles of association with GroEL. J. Biol. Chem. 27437743-37749. [DOI] [PubMed] [Google Scholar]
- 28.Kelly, C., P. Evans, L. Bergmeier, S. F. Lee, A. Progulske-Fox, A. C. Harris, A. Aitken, A. S. Bleiweis, and T. Lehner. 1989. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 258127-132. [DOI] [PubMed] [Google Scholar]
- 29.Kelly, C. G., S. Todryk, H. L. Kendal, G. H. Munro, and T. Lehner. 1995. T-cell, adhesion, and B-cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect. Immun. 633649-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, D. Y., and K. K. Kim. 2005. Structure and function of HtrA family proteins, the key players in protein quality control. J. Biochem. Mol. Biol. 38266-274. [DOI] [PubMed] [Google Scholar]
- 31.Knox, K. W., L. N. Hardy, and A. J. Wicken. 1986. Comparative studies on the protein profiles and hydrophobicity of strains of Streptococcus mutans serotype c. J. Gen. Microbiol. 1322541-2548. [DOI] [PubMed] [Google Scholar]
- 32.Kramer, G., H. Patzelt, T. Rauch, T. A. Kurz, S. Vorderwulbecke, B. Bukau, and E. Deuerling. 2004. Trigger factor peptidyl-prolyl cis/trans isomerase activity is not essential for the folding of cytosolic proteins in Escherichia coli. J. Biol. Chem. 27914165-14170. [DOI] [PubMed] [Google Scholar]
- 33.Lee, S. F., A. Progulske-Fox, and A. S. Bleiweis. 1988. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect. Immun. 562114-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemos, J. A., Y. Y. Chen, and R. A. Burne. 2001. Genetic and physiologic analysis of the groE operon and role of the HrcA repressor in stress gene regulation and acid tolerance in Streptococcus mutans. J. Bacteriol. 1836074-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemos, J. A., Y. Luzardo, and R. A. Burne. 2007. Physiologic effects of forced down-regulation of dnaK and groEL expression in Streptococcus mutans. J. Bacteriol. 1891582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loimaranta, V., N. S. Jakubovics, J. Hytonen, J. Finne, H. F. Jenkinson, and N. Stromberg. 2005. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect. Immun. 732245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyon, W. R., and M. G. Caparon. 2003. Trigger factor-mediated prolyl isomerization influences maturation of the Streptococcus pyogenes cysteine protease. J. Bacteriol. 1853661-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma, B., C. J. Tsai, and R. Nussinov. 2000. Binding and folding: in search of intramolecular chaperone-like building block fragments. Protein Eng. 13617-627. [DOI] [PubMed] [Google Scholar]
- 39.Markossian, K. A., A. A. Zamyatnin, and B. I. Kurganov. 2004. Antibacterial proline-rich oligopeptides and their target proteins. Biochemistry 691082-1091. [DOI] [PubMed] [Google Scholar]
- 40.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 401049-1057. [DOI] [PubMed] [Google Scholar]
- 41.McArthur, W. P., N. R. Rhodin, T. B. Seifert, M. W. Oli, R. A. Robinette, D. R. Demuth, and L. J. Brady. 2007. Characterization of epitopes recognized by anti-Streptococcus mutans P1 monoclonal antibodies. FEMS Immunol. Med. Microbiol. [DOI] [PubMed]
- 42.McIver, K. S., S. Subbarao, E. M. Kellner, A. S. Heath, and J. R. Scott. 1996. Identification of isp, a locus encoding an immunogenic secreted protein conserved among group A streptococci. Infect. Immun. 642548-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moisset, A., N. Schatz, Y. Lepoivre, S. Amadio, D. Wachsmann, M. Scholler, and J. P. Klein. 1994. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect. Immun. 62184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munro, G. H., P. Evans, S. Todryk, P. Buckett, C. G. Kelly, and T. Lehner. 1993. A protein fragment of streptococcal cell surface antigen I/II which prevents adhesion of Streptococcus mutans. Infect. Immun. 614590-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishihara, K., M. Kanemori, H. Yanagi, and T. Yura. 2000. Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl. Environ Microbiol. 66884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norrander, J., T. Kempe, and J. Messing. 1983. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 26101-106. [DOI] [PubMed] [Google Scholar]
- 47.Nouwen, N., G. Berrelkamp, and A. J. Driessen. 2007. Bacterial Sec-translocase unfolds and translocates a class of folded protein domains. J. Mol. Biol. 372422-433. [DOI] [PubMed] [Google Scholar]
- 48.Okahashi, N., C. Sasakawa, M. Yoshikawa, S. Hamada, and T. Koga. 1989. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol. Microbiol. 3221-228. [DOI] [PubMed] [Google Scholar]
- 49.Okahashi, N., C. Sasakawa, M. Yoshikawa, S. Hamada, and T. Koga. 1989. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol. Microbiol. 3673-678. [DOI] [PubMed] [Google Scholar]
- 50.Oli, M. W., W. P. McArthur, and L. J. Brady. 2006. A whole cell BIAcore assay to evaluate P1-mediated adherence of Streptococcus mutans to human salivary agglutinin and inhibition by specific antibodies. J. Microbiol. Methods 65503-511. [DOI] [PubMed] [Google Scholar]
- 51.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26209-221. [DOI] [PubMed] [Google Scholar]
- 52.Perry, D., and H. K. Kuramitsu. 1989. Genetic linkage among cloned genes of Streptococcus mutans. Infect. Immun. 57805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 351042-1051. [DOI] [PubMed] [Google Scholar]
- 54.Prakobphol, A., F. Xu, V. M. Hoang, T. Larsson, J. Bergstrom, I. Johansson, L. Frangsmyr, U. Holmskov, H. Leffler, C. Nilsson, T. Boren, J. R. Wright, N. Stromberg, and S. J. Fisher. 2000. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J. Biol. Chem. 27539860-39866. [DOI] [PubMed] [Google Scholar]
- 55.Randall, L. L., and S. J. Hardy. 1986. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell 46921-928. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhodin, N. R., J. M. Cutalo, K. B. Tomer, W. P. McArthur, and L. J. Brady. 2004. Characterization of the Streptococcus mutans P1 epitope recognized by immunomodulatory monoclonal antibody 6-11A. Infect. Immun. 724680-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhodin, N. R., M. L. Van Tilburg, M. W. Oli, W. P. McArthur, and L. J. Brady. 2004. Further characterization of immunomodulation by a monoclonal antibody against Streptococcus mutans antigen P1. Infect. Immun. 7213-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell, M. W., L. A. Bergmeier, E. D. Zanders, and T. Lehner. 1980. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 28486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seifert, T. B., A. S. Bleiweis, and L. J. Brady. 2004. Contribution of the alanine-rich region of Streptococcus mutans P1 to antigenicity, surface expression, and interaction with the proline-rich repeat domain. Infect. Immun. 724699-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Signas, C., G. Raucci, K. Jonsson, P. E. Lindgren, G. M. Anantharamaiah, M. Hook, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97339-347. [DOI] [PubMed] [Google Scholar]
- 63.Steffens, W. L., M. A. Goodwin, and M. B. Ard. 1997. Immunogold for detection of avian leukosis/sarcoma virus in formalin-fixed heart and kidney. Avian Pathol. 2645-52. [DOI] [PubMed] [Google Scholar]
- 64.Talay, S. R., P. Valentin-Weigand, K. N. Timmis, and G. S. Chhatwal. 1994. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol. Microbiol. 13531-539. [DOI] [PubMed] [Google Scholar]
- 65.Terleckyj, B., and G. D. Shockman. 1975. Amino acid requirements of Streptococcus mutans and other oral streptococci. Infect. Immun. 11656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694269-278. [DOI] [PubMed] [Google Scholar]
- 67.Troffer-Charlier, N., J. Ogier, D. Moras, and J. Cavarelli. 2002. Crystal structure of the V-region of Streptococcus mutans antigen I/II at 2.4 Å resolution suggests a sugar preformed binding site. J. Mol. Biol. 318179-188. [DOI] [PubMed] [Google Scholar]
- 68.Valent, Q. A., D. A. Kendall, S. High, R. Kusters, B. Oudega, and J. Luirink. 1995. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 145494-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Dolleweerd, C. J., D. Chargelegue, and J. K. Ma. 2003. Characterization of the conformational epitope of Guy's 13, a monoclonal antibody that prevents Streptococcus mutans colonization in humans. Infect. Immun. 71754-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, J., N. S. Tan, B. Ho, and J. L. Ding. 2002. Modular arrangement and secretion of a multidomain serine protease. Evidence for involvement of proline-rich region and N-glycans in the secretion pathway. J. Biol. Chem. 27736363-36372. [DOI] [PubMed] [Google Scholar]
- 71.Wen, Z. T., P. Suntharaligham, D. G. Cvitkovitch, and R. A. Burne. 2005. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect. Immun. 73219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yother, J., and D. E. Briles. 1992. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J. Bacteriol. 174601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.