Abstract
Transforming growth factor β (TGFβ) plays a dual role in oncogenesis, acting as both a tumor suppressor and a tumor promoter. These disparate processes of suppression and promotion are mediated primarily by Smad and non-Smad signaling, respectively. A central issue in understanding the role of TGFβ in the progression of epithelial cancers is the elucidation of the mechanisms underlying activation of non-Smad signaling cascades. Because the potent lipid mediator sphingosine-1-phosphate (S1P) has been shown to transactivate the TGFβ receptor and activate Smad3, we examined its role in TGFβ activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and promotion of migration and invasion of esophageal cancer cells. Both S1P and TGFβ activate ERK1/2, but only TGFβ activates Smad3. Both ligands promoted ERK1/2-dependent migration and invasion. Furthermore, TGFβ rapidly increased S1P, which was required for TGFβ-induced ERK1/2 activation, as well as migration and invasion, since downregulation of sphingosine kinases, the enzymes that produce S1P, inhibited these responses. Finally, our data demonstrate that TGFβ activation of ERK1/2, as well as induction of migration and invasion, is mediated at least in part by ligation of the S1P receptor, S1PR2. Thus, these studies provide the first evidence that TGFβ activation of sphingosine kinases and formation of S1P contribute to non-Smad signaling and could be important for progression of esophageal cancer.
Despite advances in therapeutic approaches, esophageal cancer remains one of the most lethal cancers, with an overall survival rate of 10 to 20%. There are two types of esophageal carcinoma, squamous cell and adenocarcinoma. Recent years have seen a shift in the epidemiology of this disease, manifested as an increased incidence of adenocarcinoma (19, 37). However, the ability to reverse the outcome of esophageal cancer is limited by an overall poor understanding of its biology.
Transforming growth factor β (TGFβ) plays a dual role in the development of epithelial cancers, acting as both a tumor suppressor and a tumor promoter. This dichotomy is a reflection of its multiple effects on epithelial growth and differentiation. It inhibits carcinogenesis by inducing reversible growth arrest in G1, but it promotes carcinogenesis by stimulating prometastatic processes such as migration, invasion, and epithelial mesenchymal transition (1, 34). The complexity of the biological processes impacted by TGFβ relates to its ability to activate multiple signaling pathways. The canonical Smad pathway is initiated by the ligand-induced formation of a heterodimer consisting of serine-threonine kinase TGFβ receptors I and II (TβRI and TβRII). TβRII activates TβRI, resulting in the phosphorylation of the receptor-activated Smads, i.e., Smad2 and Smad3 in the case of TGFβ1. The phosphorylated Smad alone or together with Smad4 enters the nucleus. The subsequent gene response is controlled by the interaction of Smads with both transcriptional activators and repressors, resulting in a highly cell-type-specific response (29). The growth arrest program appears to be solely dependent on this pathway (7, 30). In addition to the Smad pathway, TGFβ activates several non-Smad pathways including the extracellular signal-regulated kinase 1 and 2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), JNK, and phosphatidylinositol 3-kinase (PI-3)/Akt pathways in a cell-type-specific fashion (3, 4, 18, 20, 50). The molecular interactions linking the TβRs to these pathways are not well defined. It is important to delineate these mechanisms because, although Smad signaling plays a role in epithelial mesenchymal transition and the associated processes of migration and invasion, they are also dependent on non-Smad signaling (5, 44).
The bioactive sphingolipid mediator sphingosine-1-phosphate (S1P) is produced by two sphingosine kinase isozymes, SphK1 and SphK2. It is the ligand for a family of G protein-coupled S1P receptors 1 to 5 (S1PR1 to S1PR5), and it regulates a wide array of biological effects including growth, survival, and migration, depending on which receptors are expressed (39). There is evidence that some of the overlap in the functions of S1P and TGFβ may result from interactions between their respective signaling pathways. For example, S1P can induce the phosphorylation of Smad2 and Smad3 (36), presumably as a result of a direct interaction between S1PR1 and TβRI (21, 45). The ability to activate Smad3 is a prerequisite for S1P induction of both chemotactic migration and growth arrest in keratinocytes and chemotactic migration of Langerhans cells (33). Although it appears that S1P can directly activate the TGFβ pathway by causing TβR-mediated phosphorylation of Smads, the ability of TGFβ to directly activate S1PR-mediated signaling is less clear. In both fibroblasts and myofibroblasts, TGFβ can increase the activity of SphK1 mainly by increasing its expression 24 h after treatment (22, 47). The present study demonstrates that in cells derived from an esophageal adenocarcinoma, both S1P- and TGFβ-induced chemotactic migration and invasion require Gi-dependent ERK1/2 activation. The abilities of TGFβ to activate ERK1/2 and induce chemotactic migration and invasion also depend on rapid activation of SphK1 and formation of S1P and involve ligation of S1PR2. These studies suggest that interaction or cross talk between these pathways might play a role in the progression of esophageal cancer.
MATERIALS AND METHODS
Cell culture and reagents.
OE33 cells were purchased from the European Collection of Cell Cultures. SEG1 cells were kindly provided by Andrew Joe, Columbia University. Both cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum. TGFβ (recombinant human) was purchased from R&D Systems (Minneapolis, MN). S1P and N,N-dimethylsphingosine (DMS) were from Biomol (Plymouth Meeting, PA). Compound PD 98059 was purchased from Cell Signaling Biotechnology (Beverly, MA). Compounds JTE013 and VPC23019 were purchased from Tocris (Ellisville, MO) and Avanti Polar Lipids (Alabaster, AL), respectively. [γ-32P]ATP (3,000 Ci/mmol) was purchased from Amersham Biosciences (Pittsburgh, PA).
Immunoblotting.
Total cellular extracts were obtained and protein was quantified as previously described (8). Equal amounts of protein (30 to 50 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (Bio-Rad), and blocked for 1 h with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) (Sigma). The blots were incubated with the primary antibodies overnight at 4°C, washed three times with TBST for 10 min, and incubated with the appropriate secondary horseradish peroxidase-conjugated antibody (Zymed, San Francisco, CA) for 1 h at room temperature. Blots were then washed four times with TBST for 10 min each, developed with ECL Plus (Amersham, Piscataway, NJ), and exposed to X-Omat blue film (Kodak, Rochester, NY). Antibodies used in analyses included anti-ERK1/2 (sc154), anti-phospho ERK1/2 (sc7383), antiactin (clone sc1615), and anti-ERK1 (clone sc-93) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-p38 MAPK (9212) and anti-phospho-p38 MAPK (9211) from Cell Signaling (Beverly, MA); and anti-phospho-sphingosine kinase 1 (clone ser-225) (ECM Biosciences, Versailles, KY). Rabbit polyclonal SphK1 and SphK2 antibodies were described previously (16).
Migration and invasion.
Cells at 70% confluence were serum starved overnight and detached from culture plates by incubation with Cellstripper solution (Mediatech-Cellgro, Herndon, VA). Cells were then washed and resuspended in RPMI medium with 0.1% bovine serum albumin (BSA) at 1 × 105 cells/ml. For migration assays, cells (2 × 104 cells/well) were added to the upper chamber of Transwell chambers (Corning, Acton, MA) separated by inserts with 8-μm pores in the presence or absence of the indicated inhibitors. Following an 8-h incubation, cells were fixed in 100% methanol, washed three times in double-distilled H2O, stained with 0.1% crystal violet in phosphate-buffered saline at the ambient temperature, and then destained with double-distilled H2O. The nonmigrating cells on the upper surface of the membrane were removed with cotton swabs, and the membrane was mounted on a microscope slide. Migrating cells were counted in five randomly selected high-power fields per membrane using a light microscope, and the number of migrating cells was compared to that of simultaneously run control cells. For invasion assays, Matrigel was diluted 1:6 in RPMI medium with 0.1% BSA, 80-μl aliquots were used to coat 12-μm-pore size (12-well) Transwell chambers (Corning, Acton, MA), and allowed to gel at 37°C for 1 h. Cells (5 × 104/well) were seeded on top of the Matrigel matrix and incubated alone or with the appropriate reagents. After 24 h, the membranes were treated and counted as described above for the migration assay. Each determination represents the average of three membranes. Experiments were performed at least in triplicate.
Transfection.
ON-TARGETplus SMARTpool small interfering RNA (siRNA) against SphK1, SphK2, ERK1, and S1PR2 and control siRNAs from Dharmacon (Lafayette, CO) were introduced into cells in buffer V, using program T20 of a Nucleofector device (Amaxa, Gaithersburg, MD). Dominant negative MEK and control adenoviral vectors (Vector Biolabs, Philadelphia, PA) were introduced into cells essentially as described previously (46).
Quantitative real-time PCR.
Cells were plated at 50 to 75% confluence and cultured overnight. Total RNA was isolated using RNAEasy Plus (Qiagen) according to the manufacturer's instructions. RNA was reverse transcribed (RT) with MultiScribe (Applied Biosystems, Foster City, CA). For real-time PCR, prevalidated primer-probe sets which have equal efficiencies of amplification were purchased from Applied Biosystems (Foster City, CA). Quantitative (Q) PCRs were performed with an ABI 7900HT. ABI Prism software was used to construct a calibration curve by plotting the threshold cycle versus the logarithm of the calibrator concentration. Data were normalized to that of GAPDH.
Sphingosine kinase assays.
Cells were harvested and lysed by freeze-thawing in a buffer containing 20 mM Tris (pH 7.4), 20% glycerol, 1 mM 2-mercaptoethanol, 1 mM EDTA, 5 mM sodium orthovanadate, 40 mM glycerophosphate, 15 mM NaF, 10 g/ml leupeptin, aprotinin, and soybean trypsin inhibitor, 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM 4-deoxypyridoxine. Lysates were centrifuged at 700 × g for 10 min to remove unbroken cells. SphK1 activity was determined in the presence of 50 μM sphingosine and [γ-32P]ATP (10 μCi, 1 mM) containing MgCl2 (10 mM) in 0.25% Triton X-100, which inhibits SphK2, as described previously (28). SphK2 activity was determined with sphingosine added as a complex with 4 mg/ml BSA and [γ-32P]ATP in the presence of 1 M KCl, conditions under which SphK2 activity is optimal and SphK1 is strongly inhibited (28). Labeled S1P was extracted and separated by thin-layer chromatography on silica gel G60, with chloroform-acetone-methanol-acetic acid-H2O (10:4:3:2:1 [vol/vol]) as solvent. Radioactive bands corresponding to S1P were quantified with an FX Molecular Imager device (Bio-Rad). SphK-specific activity is expressed as pmol of S1P formed per min per mg protein.
Measurement of S1P.
Cells (4 × 105) were treated as indicated and harvested in 25 mM HCl-methanol. The levels of S1P were determined exactly as previously described (9). Briefly, the assay utilizes an alkaline lipid extraction to selectively separate S1P from sphingosine and other phospholipids. Extracted S1P is efficiently converted to sphingosine by alkaline phosphatase treatment. Sphingosine thus formed is quantitated by conversion to [32P]S1P, with recombinant SphK1 as described previously (9). [32P]S1P was extracted and then separated by thin-layer chromatography on silica gel G60, with chloroform-acetone-methanol-acetic acid-H2O (10:4:3:2:1 [vol/vol]) as solvent. Radioactive bands corresponding to S1P were quantified with an FX Molecular Imager device (Bio-Rad).
Statistical analysis.
Statistical significance was determined by Student's t test. P values of <0.05 were considered significant.
RESULTS
TGFβ and S1P induce ERK1/2-dependent chemotactic migration and invasion.
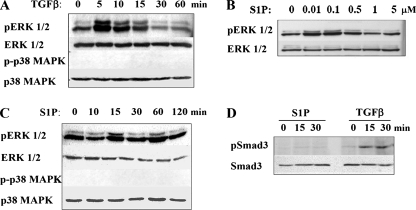
Previous studies demonstrated that in the majority of esophageal carcinoma-derived cell lines, the TGFβ pathway is intact and functional (24). Although the ability to activate Smads is universal among cells with functional TGFβ pathways, the ability to activate different MAPK pathways is cell type dependent (3, 4, 20). To determine which MAPK pathways are activated in esophageal adenocarcinomas, the ability of TGFβ to activate MAPK pathways was assessed with OE33 cells, which were derived from an esophageal adenocarcinoma that developed in the context of Barrett's dysplasia (35). In these cells, TGFβ induced phosphorylation of ERK1/2 but not p38 MAPK within 5 min (Fig. 1A). The rapid activation of ERK1/2 suggests that it is Smad independent. The biochemical mechanisms underlying Smad-independent activation of MAPK pathways are poorly understood. One possible explanation is that this occurs as a consequence of cross talk with other pathways. Several studies indicate that S1P, which has some biologic actions similar to those of TGFβ, by binding to S1P receptors can activate Smads via the transactivation of TβRs (33, 36, 45). To ascertain the potential for interaction between these pathways in epithelial cancer cells, SIP activation of MAPKs was examined with OE33 cells. S1P induced a dose-dependent activation of ERK1/2 but not of p38 MAPK (Fig. 1B and C). Interestingly, S1P did not induce Smad3 phosphorylation in these cells (Fig. 1D).
FIG. 1.
Activation of MAP kinases by TGFβ and S1P. OE33 cells were treated with 80 pM TGFβ for the indicated times (A), with the indicated concentrations of S1P (μM) for 15 min (B), with 50 nM S1P for the indicated length of time (C), or with either 80 pM TGFβ or 50 nM S1P for 0, 15, and 30 min (D). Cells were lysed, and equal amounts of lysates were separated by SDS-PAGE. Activation of ERK1/2, p38, and Smad3 was determined by immunoblotting with phospho-specific antibodies as indicated. Blots were stripped and reprobed with ERK1/2, p38, or Smad3 antibodies to demonstrate equal loading.
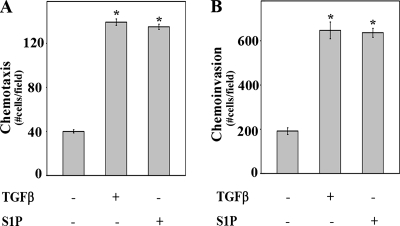
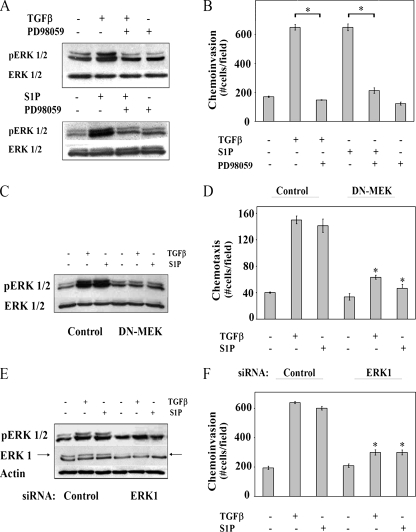
Non-Smad TGFβ signaling is associated with promoting metastasis by inducing cellular changes that lead to migration and invasion (3, 44), and it is well established that activation of the ERK1/2 pathways plays a role in these processes (20, 44). Both TGFβ and S1P stimulated chemotactic migration and invasion of OE33 to similar extents (Fig. 2A and B). Chemoinvasion induced by both ligands was blocked by PD98059, a pharmacologic inhibitor of MEK (Fig. 3A and B). In addition, the introduction of a dominant-negative form of MEK to block activation of ERK1/2, as well as to downregulate ERK1 expression with a specific siRNA, inhibited chemotactic migration and invasion (Fig. 3C to F), confirming the importance of activation of the ERK pathway in chemotactic migration and invasion induced by TGFβ and S1P.
FIG. 2.
TGFβ and S1P stimulate chemotactic migration (chemotaxis) and invasion to similar extents. (A) Transwell migration assays were performed as described in Materials and Methods. Medium without or with TGFβ (80 pM) or S1P (50 nM) was added to the lower chamber as indicated. Data are means ± standard errors of the means from three separate experiments. Asterisk, P < 0.05. (B) Invasion assays were performed with Matrigel-coated membranes as described in Materials and Methods. Medium, without or with TGFβ (80 pM) or S1P (50 nM), was added to the lower chambers as indicated. Data are means ± standard errors of the means from three separate experiments. Asterisk, P < 0.05.
FIG. 3.
Chemotactic migration and invasion induced by TGFβ or S1P is ERK1/2 dependent. (A) OE33 cells were incubated for 15 min in the presence or absence of TGFβ (80 pM), S1P (50 nM), and the MEK inhibitor PD98059 (50 μM) as indicated. Cells were lysed, and activation of ERK1/2 was determined by immunoblotting equal amounts of lysates with anti-phospho ERK1/2. Blots were stripped and reprobed with anti-ERK1/2 as a loading control. (B) Transwell invasion assays were performed as described in Materials and Methods. PD98059 (50 μM) was added to the upper chambers, and either TGFβ (80 pM) or S1P (50 nM) was in the lower chamber. OE33 cells were transduced with either control or dominant negative MEK (DN-MEK) adenoviral vectors (C and D) or transfected with control or ERK1 siRNAs (E and F). (C and E) Cells were analyzed by Western blotting as in panel A, except that blots were also stripped and reprobed with actin as a control for equal loading and transfer. Chemotactic migration (D) and invasion (F) assays were performed as described in Materials and Methods. Data are means ± standard errors of the means from three separate experiments. Asterisk, P < 0.05.
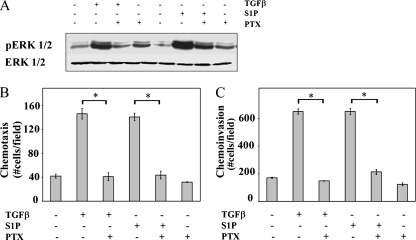
S1P activates multiple signaling pathways, including ERK1/2, via G protein-coupled receptors (39). In agreement with results of many other cell types (12, 40, 43), pertussis toxin pretreatment, which inhibits Gi, inhibited ERK1/2 activation induced by S1P in OE33 cells (Fig. 4A). Surprisingly, pertussis toxin also inhibited the activation of ERK1/2 by TGFβ (Fig. 4A). Moreover, pertussis toxin not only inhibited S1P-induced chemotactic migration (Fig. 4B) and invasion (Fig. 4C), it also blocked the ability of TGFβ to induce these processes. Collectively, these data indicate that both S1P- and TGFβ-induced activation of ERK1/2, as well as induction of chemotactic migration and invasion, are Gi-dependent processes.
FIG. 4.
ERK1/2 activation, chemotactic migration, and chemotactic invasion are Gi dependent (A to C). OE33 cells were pretreated for 2 h with 100 ng/ml pertussis toxin as indicated. (A) Cells were treated with TGFβ (80 pM) or S1P (50 nM) for 15 min. Cell lysates were separated by SDS-PAGE and analyzed for pERK1/2 and total ERK1/2 by Western analysis. Chemotactic migration (B) and invasion (C) assays were performed as described in Materials and Methods. Data are means ± standard errors of the means from triplicate cultures. Asterisk, P < 0.05.
TGFβ-induced migration and invasion require SphK.
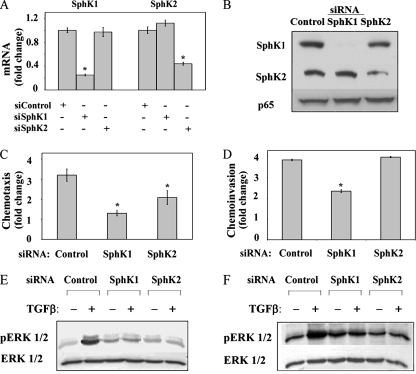
The overlap of the activation of signaling pathways and the biologic responses between S1P and TGFβ raised the possibility that cross talk between these pathways plays a role in TGFβ activation of ERK1/2 and induction of migration and invasion. To address this possibility, we first examined the effect of the pan-SphK inhibitor DMS on TGFβ responses. Pretreatment of OE33 cells with DMS inhibited TGFβ activation of ERK1/2, as well as chemotactic migration and invasion (Fig. 5), suggesting that SphK activity contributes to these processes. Since both SphK1 and SphK2 are expressed in OE33 cells (Fig. 6A and B), either or both isoenzymes could be involved. Consequently, to examine the role of these SphKs in TGFβ-induced chemotactic migration and invasion, the expression of each isozyme was decreased by using SphK isozyme-specific siRNAs (Fig. 6 A and B). SphK1 siRNA reduced both the SphK1 mRNA and the protein levels by >70% and had no effect on SphK2 expression. The SphK2 siRNA was equally specific but somewhat less efficient at reducing SphK2 expression. Decreasing SphK1 expression significantly decreased both TGFβ-induced chemotactic migration and invasion, whereas decreasing SphK2 expression inhibited chemotactic migration less effectively and had no effect on chemotactic invasion (Fig. 6C and D). Decreased expression of SphKs also inhibited TGFβ activation of ERK1/2 (Fig. 6E). To confirm the role of SphKs in ERK1/2 activation in esophageal cancer cells, SphK levels were modulated in a second esophageal adenocarcinoma-derived cell line, SEG1. The TGFβ receptors and Smad2, Smad3, and Smad4 are present and functional in this cell line, although Smad-dependent signaling is suppressed due to a defect in the metabolism of the SnoN oncoprotein (8, 24, 27). Similar to results with OE33 cells, the downregulation of SphK1 or SphK2 blocked ERK1/2 activation in SEG1 cells (Fig. 6F).
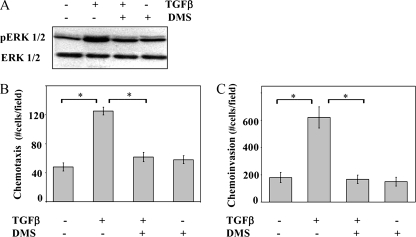
FIG. 5.
The SphK inhibitor DMS blocks TGFβ-induced ERK1/2 activation and cell motility. (A) OE33 cells were incubated for 15 min in the absence or presence of TGFβ (80 pM) and DMS (5 μM) as indicated, and ERK1/2 activation was determined by sequential immunoblotting with anti-phospho ERK1/2 and anti-ERK1/2. Chemotactic migration (B) and invasion (C) assays were performed as described in Materials and Methods. TGFβ (80 pM) and DMS (5 μM) were added to the lower and upper chambers, respectively. Data are means ± standard errors of the means from three separate experiments. Asterisk, P < 0.05.
FIG. 6.
SphK1 and SphK2 have different roles in TGFβ-induced chemotactic migration and invasion. OE33 cells were transfected with control, SphK1, or SphK2 siRNA as indicated. (A) RNA was isolated and reverse transcribed, and SphK1, SphK2 and GAPDH levels were measured by QRT-PCR. SphKs are normalized to GAPDH. Data are expressed as the change with respect to control siRNA. (B) Total cell lysates were assayed for the expression of the indicated proteins by immunoblotting with anti-SphK1 and anti-SphK2 antibodies. Blots were also probed with anti-p65 to show equal loading. Chemotactic migration (C) and invasion (D) were determined, and data were expressed as the change in the ratio of migrating cells in TGFβ treated to untreated cells for each group of transfectants. The results are means ± standard errors of the means from three separate experiments. Asterisk, P < 0.05. OE33 cells (E) or SEG1 cells (F) were transfected with the indicated siRNAs and cultured without or with TGFβ (80 pM) as indicated. The activation of ERK1/2 was determined by immunoblotting with pERK1/2 and ERK1/2 as loading controls.
TGFβ activates SphKs to generate S1P.
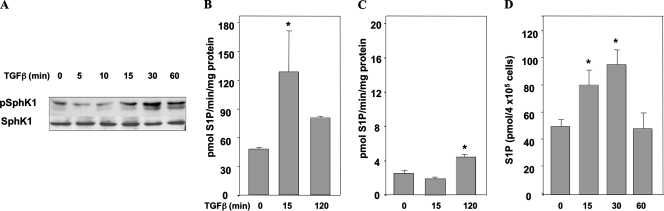
Previous studies of primary human fibroblasts demonstrated that TGFβ increased transcription and activity of SphK1 (47). TGFβ-induced differentiation of myofibroblasts has also been linked to the increase in SphK activity that occurs 24 h after stimulation (22). Since both chemotactic migration and invasion occur more rapidly, activation of SphKs by TGFβ in OE33 cells is likely to be more rapid. In fact, within 15 min, TGFβ induced phosphorylation of SphK1 at Ser225 (Fig. 7A), which has been shown to be critical for SphK1 activation (32). In addition, isozyme-specific SphK assays (28) revealed that TGFβ increases the activity of both isozymes. However, SphK1 accounted for most of the SphK activity, since there was a very low level of SphK2 activity (Fig. 7B and C). There was a rapid increase in SphK1 activity, which was detected 15 min after treatment with TGFβ, whereas SphK2 was activated with slower kinetics. Importantly, TGFβ induced a significant increase in cellular levels of S1P within 15 min (Fig. 7D).
FIG. 7.
TGFβ activates SphK to generate S1P. (A) OE33 cells were treated with TGFβ (80 pM) for the indicated time. Activation of SphK1 was determined by immunoblotting with anti-phospho Ser225. Blots were stripped and reprobed with anti-SphK1 antibody to confirm equal loading and transfer. (B and C) OE33 cells were treated with TGFβ for the indicated times and lysed, and SphK1 (B) and SphK2 (C) activities were determined with isoenzyme-specific assays. SphK activity is expressed as pmol/min/mg protein. (D) S1P mass levels were measured in duplicate cultures of 4 × 105 cells as described in Materials and Methods and expressed as pmol. Data are means ± standard errors of the means. Asterisk, P < 0.05.
S1PR2 ligation is involved in TGFβ-induced migration and invasion.
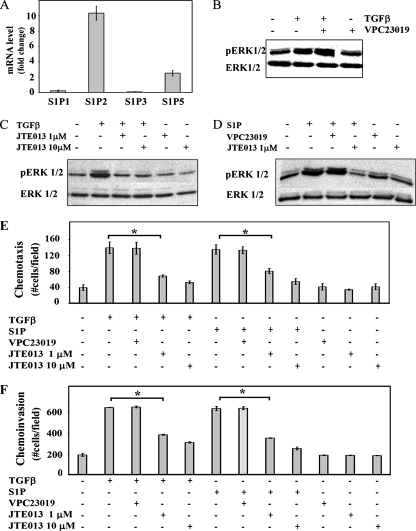
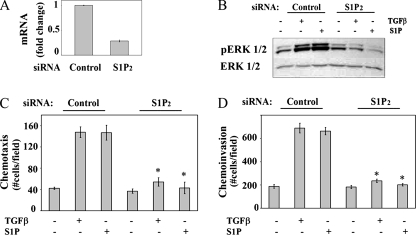
Activation of SphKs leads to increased intracellular S1P, which can either act as a second messenger or exit the cell and act as a ligand for G protein-coupled receptors S1PR1 to S1PR5 (15). To examine these possibilities, the effect of inhibiting S1P receptors on the ability of TGFβ to activate ERK1/2 and mediate chemotactic migration and invasion was determined. In OE33 cells, there are low (S1PR1 and S1PR3) to nondetectable (S1PR4) levels of mRNA for some S1P receptors, whereas mRNAs for S1PR2 and S1PR5 are present at higher levels (Fig. 8A). To ascertain if these S1P receptors were involved, OE33 cells were stimulated with TGFβ in the presence of JTE013, an antagonist of S1PR2, or VPC23019, an antagonist of S1PR1 and S1PR3, at 10 μM (6, 31). JTEO13, but not VPC23019, blocked both the TGFβ and the S1P activation of ERK1/2 (Fig. 8B, C, and D). Furthermore, JTE013, but not VPC23019, also blocked both S1P- and TGFβ-induced chemotactic migration and significantly inhibited S1P- and TGFβ-induced chemotactic invasion (Fig. 8E and F). These results suggest that ligation of S1PR2 by S1P is critical for these TGFβ responses. The specificity of this pharmacological approach was further confirmed by the downregulation of S1PR2 with a specific siRNA targeted to this receptor. Transfection of OE33 cells with S1PR2 siRNA decreased its expression by 70% (Fig. 9A) without a significant reduction in expression of the other S1P receptors (data not shown). Decreasing expression of S1PR2 also significantly inhibited both the S1P- and the TGFβ-induced activation of ERK1/2 (Fig. 9B), as well as chemotactic migration and invasion (Fig. 9C and D). In sum, these findings demonstrate that TGFβ activates ERK1/2 in a S1PR2-dependent fashion and that S1PR2 is involved in migration and invasion of OE33 cells induced by TGFβ.
FIG. 8.
S1PR2 receptor activation is involved in TGFβ-induced ERK1/2 activation, as well as in chemotactic migration and invasion. (A) S1P receptor mRNA expression in OE33 cells. RNA was isolated and reverse transcribed, and S1PR1 to S1PR5 and GADPH levels were measured by QRT-PCR and normalized to those of GAPDH. Data are expressed relative to S1PR1 mRNA. (B, C, and D) OE33 cells were treated for 15 min without or with TGFβ (80 pM), S1P (50 nM), VPC23019 (10 μM), and JTE013 (JTE013, 1 or 10 μM) as indicated. Activation of ERK1/2 was determined by immunoblotting with pERK1/2 antibody. Blots were stripped and reprobed with anti-ERK1/2 antibody to demonstrate equal loading and transfer. Chemotactic migration (E) and invasion (F) of OE33 induced by 80 pM TGFβ or 50 nM S1P were determined in the absence or presence of VPC23019 (10 μM) and the indicated concentrations of JTE013. Data are means ± standard errors of the means from three separate experiments. Asterisk, P < 0.05.
FIG. 9.
S1PR2 ligation is involved in TGFβ-induced ERK1/2 activation, as well as in chemotactic migration and invasion. OE33 cells were transfected with siControl or siS1PR2 RNAs as indicated. (A) RNA was isolated and reverse transcribed, and S1P2 and GAPDH levels were measured by QRT-PCR. Data are expressed as changes after normalization to GAPDH. (B) OE33 cells were treated with TGFβ (80 pM) or S1P (50 nM) for 15 min. Cell lysates were separated by SDS-PAGE and analyzed for pERK1/2 and total ERK1/2 by Western analysis. Chemotactic migration (C) and invasion (D) assays were performed as described in Materials and Methods.
DISCUSSION
In addition to the Smad pathway, TGFβ activates MAPK pathways in a cell type-dependent fashion (3, 4, 18, 20). The activation of these pathways occurs with various kinetics and can be either Smad dependent or Smad independent. For example, in hepatocytes (48) and fibroblasts (2), TGFβ activation of p38 MAPK occurs after several hours. This delayed activation of p38 MAPK activation requires Smad induction of Gadd45B expression (48) and is, thus, not Smad independent. However, MAPK activation can also occur rapidly and with kinetics that parallel those of Smad activation (17, 23, 38, 42), suggesting that the activation of these pathways can also be Smad independent. In OE33 esophageal adenocarcinoma cells, TGFβ activation of ERK1/2 occurs rapidly and, thus, is likely Smad independent.
The molecular mechanisms for Smad-independent activation of non-Smad pathways by TGFβ ligation of its receptors are poorly understood. Potentially, they involve a combination of pathway activation by TβR interactions with scaffold proteins (25) and cross talk with other receptors. In regard to the latter, several studies have demonstrated that S1P induces phosphorylation of Smad3 as a result of cross talk between S1PR3 and TGFβ receptors (33, 36, 45). In OE33 cells, S1P, like TGFβ, rapidly activated ERK1/2 but not p38MAPK. Interestingly, S1P does not stimulate Smad phosphorylation in these cells. OE33 cells express both S1PR2 and S1PR5 but not the other S1P receptors. Thus, unlike S1PR3, S1PR2 appears to be unable to interact with TGFβ receptors to activate Smad-mediated signaling.
Since it is well established that the activation of MAPK pathways plays a central role in the protumorigenic effects of TGFβ (44), delineating the mechanisms underpinning their activation is important for understanding the role of TGFβ in the progression of epithelial cancers. Our observation that both S1P and TGFβ stimulated migration and invasion in an ERK1/2-dependent fashion, together with our finding that both S1P and TGFβ mediation of these processes is Gi protein dependent, raised the possibility that cross talk between TGFβ and S1P signaling pathways is involved in TGFβ activation of ERK1/2 and the associated biologic processes. This idea is supported by the observation that TGFβ activation of ERK1/2, as well as its ability to stimulate migration, is abrogated by the SphK inhibitor DMS, which prevents the generation of intracellular S1P (10). Both SphK1 and SphK2 are expressed in OE33 cells, and TGFβ activation of ERK1/2 is sensitive to inhibition or downregulation of these isozymes. However, they play different roles in TGFβ-induced migration and invasion. Similar to previous results showing that both SphK1 and SphK2 are required for migration of breast cancer cells toward EGF (16), both SphKs were required for TGFβ-induced migration of OE33 cells, although migration was far more sensitive to decreases in the activity of SphK1 than that of SphK2. SphK1, but not SphK2, was required for invasion, indicating that SphK1 and SphK 2 may play different roles in the protumorigenic effects of TGFβ in esophageal cancer.
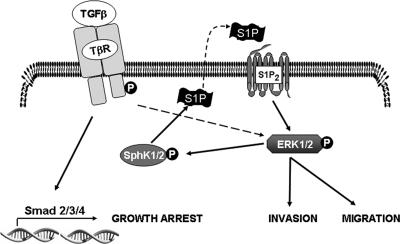
Although several agonists have been shown to activate SphK1, and a few have been shown to activate both SphK1 and SphK2 (reviewed in reference 15), previous studies indicated that in fibroblasts, TGFβ simulated SphK1 but not SphK2 and that it did so indirectly by increasing expression of SphK1 protein over a prolonged period of time (47). Similarly, SphK expression in myofibroblasts was increased by TGFβ at 24 h (22). However, TGFβ increased intracellular S1P more rapidly in these cells (22), suggesting that it may also activate SphK1 in them. In agreement, TGFβ rapidly and transiently stimulated phosphorylation of SphK1 in OE33 cells, which was accompanied by parallel increases in enzymatic activity. Increases in activity levels of SphK1 and SphK2 were independent of effects on protein expression, as TGFβ did not alter levels of mRNA for either SphK1 or SphK2 within 3 h (data not shown). Since both SphK1 and SphK2 have been shown to be phosphorylated and activated by ERK1/2 in other types of cells (14, 32) and since TGFβ activates ERK1/2 in OE33 cells, our results suggest that activation of ERK1/2 by TGFβ might be responsible for the increased activity of SphK1 and SphK2 in these cells. This is an intriguing possibility since we found that SphK1 and SphK2 are required for the TGFβ activation of ERK1/2. Therefore, some level of SphK-independent, TGFβ-mediated ERK1/2 activation, possibly resulting from TβRI phosphorylation of Shc to activate ERK1/2 (25), may occur. TGFβ activation of SphK1 would then further enhance ERK1/2 activation via an autocrine mechanism involving S1PR2 engagement following S1P release (Fig. 10). If so, SphK1 would constitute an important component of a positive feedback loop that may modulate the amplitude and duration of ERK1/2 activation in response to TGFβ.
FIG. 10.
Model of cross talk between TGFβ and S1P signaling pathways. See text for more details.
It has previously been demonstrated that intracellularly generated S1P can exit the cells and activate its receptors in an autocrine or paracrine manner (15). Consistent with this “inside-out” signaling, treatment of OE33 cells with an S1PR2 antagonist inhibited both TGFβ activation of ERK1/2 and induction of chemotactic migration and invasion. Each of the S1P receptors couples with specific G proteins and mediates distinct functions (39). Generally, activation of S1PR1 and S1PR3, neither of which was detected in OE33 cells, is associated with promoting cellular motility (11, 26, 41). However, S1PR2 is usually considered to be a repellant receptor and has been shown to inhibit migration toward PDGF and other growth factors primarily by the G12/13-dependent suppression of Rac (13, 40). Interestingly, we found that S1PR2 was required for S1P and TGFβ induction of chemotactic migration in OE33 cells. Consistent with our results, the overexpression of Gi reversed S1PR2-mediated, G12/13-dependent suppression of Rac and migration (40). It is likely that the integration of Gi-, G12/13-, or Gq-dependent positive and negative regulatory signals determines the migratory activity of S1PR2. Hence, in OE33 cells, S1PR2 must be coupled mainly to Gi. S1PR2 is also important for S1P- or TGFβ-induced invasion of OE33 cells. Similarly, a S1PR2 knockdown significantly decreased invasion of U-373 MG glioblastoma cells through Matrigel (49). Thus, S1P stimulation of S1PR2 might positively affect invasion.
Taken together, the studies presented here provide evidence for a new paradigm for TGFβ activation of non-Smad signaling and induction of migration and invasion (Fig. 10). In this scheme, ligation of the TGFβ receptor activates SphKs, resulting in increased levels of cellular S1P that can exit the cell and act in a paracrine and/or autocrine manner to ligate S1PR2. In turn, activated S1PR2 coupled mainly to Gi further activates ERK1/2, which is necessary for TGFβ-induced migration and invasion. This cross talk between the TGFβ and S1P signaling pathways amplifies TGFβ signaling and could play an important role in the progression of esophageal cancer.
Acknowledgments
This work was supported in part by Philip Morris, USA Inc. and Philip Morris International (to D.A.L.) and by NIH grant R37GM043880 (to S.S.).
We thank Paul Dent for kindly providing the dominant negative MEK and control adenoviral vectors.
Footnotes
Published ahead of print on 21 April 2008.
REFERENCES
- 1.Akhurst, R. J., and R. Derynck. 2001. TGF-beta signaling in cancer: a double-edged sword. Trends Cell Biol. 11S44-S51. [DOI] [PubMed] [Google Scholar]
- 2.Bakin, A. V., C. Rinehart, A. K. Tomlinson, and C. L. Arteaga. 2002. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 1153193-3206. [DOI] [PubMed] [Google Scholar]
- 3.Bakin, A. V., A. K. Tomlinson, N. A. Bhowmick, H. L. Moses, and C. L. Arteaga. 2000. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 27536803-36810. [DOI] [PubMed] [Google Scholar]
- 4.Bhowmick, N. A., R. Zent, M. Ghiassi, M. McDonnell, and H. L. Moses. 2001. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 27646707-46713. [DOI] [PubMed] [Google Scholar]
- 5.Davies, M., M. Robinson, E. Smith, S. Huntley, S. Prime, and I. Paterson. 2005. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J. Cell Biochem. 95918-931. [DOI] [PubMed] [Google Scholar]
- 6.Davis, M. D., J. J. Clemens, T. L. Macdonald, and K. R. Lynch. 2005. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 2809833-9841. [DOI] [PubMed] [Google Scholar]
- 7.de Caestecker, M. P., E. Piek, and A. B. Roberts. 2000. Role of transforming growth factor-beta signaling in cancer. J. Natl. Cancer Inst. 921388-1402. [DOI] [PubMed] [Google Scholar]
- 8.Edmiston, J. S., W. A. Yeudall, T. D. Chung, and D. A. Lebman. 2005. Inability of transforming growth factor-beta to cause SnoN degradation leads to resistance to transforming growth factor-beta-induced growth arrest in esophageal cancer cells. Cancer Res. 654782-4788. [DOI] [PubMed] [Google Scholar]
- 9.Edsall, L. C., and S. Spiegel. 1999. Enzymatic measurement of sphingosine 1-phosphate. Anal. Biochem. 27280-86. [DOI] [PubMed] [Google Scholar]
- 10.Edsall, L. C., J. R. Van Brocklyn, O. Cuvillier, B. Kleuser, and S. Spiegel. 1998. N,N-dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 3712892-12898. [DOI] [PubMed] [Google Scholar]
- 11.English, D., Z. Welch, A. T. Kovala, K. Harvey, O. V. Volpert, D. N. Brindley, and J. G. Garcia. 2000. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 142255-2265. [DOI] [PubMed] [Google Scholar]
- 12.Gonda, K., H. Okamoto, N. Takuwa, Y. Yatomi, H. Okazaki, T. Sakurai, S. Kimura, R. Sillard, K. Harii, and Y. Takuwa. 1999. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem. J. 33767-75. [PMC free article] [PubMed] [Google Scholar]
- 13.Goparaju, S. K., P. S. Jolly, K. R. Watterson, M. Bektas, S. Alvarez, S. Sarkar, L. Mel, I. Ishii, J. Chun, S. Milstien, and S. Spiegel. 2005. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol. Cell. Biol. 254237-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hait, N. C., A. Bellamy, S. Milstien, T. Kordula, and S. Spiegel. 2007. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 28212058-12065. [DOI] [PubMed] [Google Scholar]
- 15.Hait, N. C., C. A. Oskeritzian, S. W. Paugh, S. Milstien, and S. Spiegel. 2006. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta 17582016-2026. [DOI] [PubMed] [Google Scholar]
- 16.Hait, N. C., S. Sarkar, H. Le Stunff, A. Mikami, M. Maceyka, S. Milstien, and S. Spiegel. 2005. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J. Biol. Chem. 28029462-29469. [DOI] [PubMed] [Google Scholar]
- 17.Hanafusa, H., J. Ninomiya-Tsuji, N. Masuyama, M. Nishita, J. Fujisawa, H. Shibuya, K. Matsumoto, and E. Nishida. 1999. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J. Biol. Chem. 27427161-27167. [DOI] [PubMed] [Google Scholar]
- 18.Hartsough, M. T., and K. M. Mulder. 1995. Transforming growth factor beta activation of p44 mapk in proliferating cultures of epithelial cells. J. Biol. Chem. 2707117-7124. [DOI] [PubMed] [Google Scholar]
- 19.Holmes, R. S., and T. L. Vaughan. 2007. Epidemiology and pathogenesis of esophageal cancer. Semin. Radiat. Oncol. 172-9. [DOI] [PubMed] [Google Scholar]
- 20.Javelaud, D., and A. Mauviel. 2005. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 245742-5750. [DOI] [PubMed] [Google Scholar]
- 21.Keller, C. D., G. P. Rivera, M. Tolle, M. van der Giet, J. Chun, H. H. Radeke, M. Schafer-Korting, and B. Kleuser. 2007. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am. J. Pathol. 170281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kono, Y., T. Nishiuma, Y. Nishimura, Y. Kotani, T. Okada, S. I. Nakamura, and M. Yokoyama. 2007. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF{beta}1. Am. J. Respir. Cell Mol. Biol. 397395-404. [DOI] [PubMed] [Google Scholar]
- 23.Kwak, H. J., M. J. Park, H. Cho, C. M. Park, S. I. Moon, H. C. Lee, I. C. Park, M. S. Kim, C. H. Rhee, and S. I. Hong. 2006. Transforming growth factor-beta1 induces tissue inhibitor of metalloproteinase-1 expression via activation of extracellular signal-regulated kinase and Sp1 in human fibrosarcoma cells. Mol. Cancer Res. 4209-220. [DOI] [PubMed] [Google Scholar]
- 24.Lebman, D. A., J. S. Edmiston, T. D. Chung, and S. R. Snyder. 2002. Heterogeneity in the transforming growth factor beta response of esophageal cancer cells. Int. J. Oncol. 201241-1246. [PubMed] [Google Scholar]
- 25.Lee, M. K., C. Pardoux, M. C. Hall, P. S. Lee, D. Warburton, J. Qing, S. M. Smith, and R. Derynck. 2007. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 263957-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, O. H., Y. M. Kim, Y. M. Lee, E. J. Moon, D. J. Lee, J. H. Kim, K. W. Kim, and Y. G. Kwon. 1999. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 264743-750. [DOI] [PubMed] [Google Scholar]
- 27.Levy, L., M. Howell, D. Das, S. Harkin, V. Episkopou, and C. S. Hill. 2007. Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol. Cell. Biol. 276068-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, H., M. Sugiura, V. E. Nava, L. C. Edsall, K. Kono, S. Poulton, S. Milstien, T. Kohama, and S. Spiegel. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 27519513-19520. [DOI] [PubMed] [Google Scholar]
- 29.Massague, J., and Y. G. Chen. 2000. Controlling TGF-beta signaling. Genes Dev. 14627-644. [PubMed] [Google Scholar]
- 30.Matsuura, I., N. G. Denissova, G. Wang, D. He, J. Long, and F. Liu. 2004. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430226-231. [DOI] [PubMed] [Google Scholar]
- 31.Osada, M., Y. Yatomi, T. Ohmori, H. Ikeda, and Y. Ozaki. 2002. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem. Biophys. Res. Commun. 299483-487. [DOI] [PubMed] [Google Scholar]
- 32.Pitson, S. M., P. A. Moretti, J. R. Zebol, H. E. Lynn, P. Xia, M. A. Vadas, and B. W. Wattenberg. 2003. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 225491-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radeke, H. H., H. von Wenckstern, K. Stoidtner, B. Sauer, S. Hammer, and B. Kleuser. 2005. Overlapping signaling pathways of sphingosine 1-phosphate and TGF-beta in the murine Langerhans cell line XS52. J. Immunol. 1742778-2786. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, A. B., and L. M. Wakefield. 2003. The two faces of transforming growth factor beta in carcinogenesis. Proc. Natl. Acad. Sci. USA 1008621-8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockett, J. C., K. Larkin, S. J. Darnton, A. G. Morris, and H. R. Matthews. 1997. Five newly established oesophageal carcinoma cell lines: phenotypic and immunological characterization. Br. J. Cancer 75258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauer, B., R. Vogler, H. von Wenckstern, M. Fujii, M. B. Anzano, A. B. Glick, M. Schafer-Korting, A. B. Roberts, and B. Kleuser. 2004. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J. Biol. Chem. 27938471-38479. [DOI] [PubMed] [Google Scholar]
- 37.Siewert, J. R., and K. Ott. 2007. Are squamous and adenocarcinomas of the esophagus the same disease? Semin. Radiat. Oncol. 1738-44. [DOI] [PubMed] [Google Scholar]
- 38.Sowa, H., H. Kaji, T. Yamaguchi, T. Sugimoto, and K. Chihara. 2002. Activations of ERK1/2 and JNK by transforming growth factor beta negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J. Biol. Chem. 27736024-36031. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel, S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4397-407. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto, N., N. Takuwa, H. Okamoto, S. Sakurada, and Y. Takuwa. 2003. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol. Cell. Biol. 231534-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, F., J. R. Van Brocklyn, J. P. Hobson, S. Movafagh, Z. Zukowska-Grojec, S. Milstien, and S. Spiegel. 1999. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 27435343-35350. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe, H., M. P. de Caestecker, and Y. Yamada. 2001. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem. 27614466-14473. [DOI] [PubMed] [Google Scholar]
- 43.Windh, R. T., M. J. Lee, T. Hla, S. An, A. J. Barr, and D. R. Manning. 1999. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J. Biol. Chem. 27427351-27358. [DOI] [PubMed] [Google Scholar]
- 44.Xie, L., B. K. Law, A. M. Chytil, K. A. Brown, M. E. Aakre, and H. L. Moses. 2004. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 6603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin, C., S. Ren, B. Kleuser, S. Shabahang, W. Eberhardt, H. Radeke, M. Schafer-Korting, J. Pfeilschifter, and A. Huwiler. 2004. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J. Biol. Chem. 27935255-35262. [DOI] [PubMed] [Google Scholar]
- 46.Yacoub, A., W. Hawkins, D. Hanna, H. Young, M. A. Park, M. Grant, J. D. Roberts, D. T. Curiel, P. B. Fisher, K. Valerie, S. Grant, M. P. Hagan, and P. Dent. 2007. Human chorionic gonadotropin modulates prostate cancer cell survival after irradiation or HMG CoA reductase inhibitor treatment. Mol. Pharmacol. 71259-275. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka, M., D. Shegogue, H. Pei, S. Bu, A. Bielawska, J. Bielawski, B. Pettus, Y. A. Hannun, L. Obeid, and M. Trojanowska. 2004. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta and mediates TIMP-1 up-regulation. J. Biol. Chem. 27953994-54001. [DOI] [PubMed] [Google Scholar]
- 48.Yoo, J., M. Ghiassi, L. Jirmanova, A. G. Balliet, B. Hoffman, A. J. Fornace, Jr., D. A. Liebermann, E. P. Bottinger, and A. B. Roberts. 2003. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J. Biol. Chem. 27843001-43007. [DOI] [PubMed] [Google Scholar]
- 49.Young, N., and J. R. Van Brocklyn. 2007. Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P2 on cell migration and invasiveness. Exp. Cell Res. 3131615-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zavadil, J., M. Bitzer, D. Liang, Y. C. Yang, A. Massimi, S. Kneitz, E. Piek, and E. P. Bottinger. 2001. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc. Natl. Acad. Sci. USA 986686-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]