Abstract
Cysteinyl leukotrienes (cys-LTs) are potent inflammatory lipid mediators, of which leukotriene (LT) E4 is the most stable and abundant in vivo. Although only a weak agonist of established G protein-coupled receptors (GPCRs) for cys-LTs, LTE4 potentiates airway hyper-responsiveness (AHR) by a cyclooxygenase (COX)-dependent mechanism and induces bronchial eosinophilia. We now report that LTE4 activates human mast cells (MCs) by a pathway involving cooperation between an MK571-sensitive GPCR and peroxisome proliferator-activated receptor (PPAR)γ, a nuclear receptor for dietary lipids. Although LTD4 is more potent than LTE4 for inducing calcium flux by the human MC sarcoma line LAD2, LTE4 is more potent for inducing proliferation and chemokine generation, and is at least as potent for upregulating COX-2 expression and causing prostaglandin D2 (PGD2) generation. LTE4 caused phosphorylation of extracellular signal-regulated kinase (ERK), p90RSK, and cyclic AMP-regulated-binding protein (CREB). ERK activation in response to LTE4, but not to LTD4, was resistant to inhibitors of phosphoinositol 3-kinase. LTE4-mediated COX-2 induction, PGD2 generation, and ERK phosphorylation were all sensitive to interference by the PPARγ antagonist GW9662 and to targeted knockdown of PPARγ. Although LTE4-mediated PGD2 production was also sensitive to MK571, an antagonist for the type 1 receptor for cys-LTs (CysLT1R), it was resistant to knockdown of this receptor. This LTE4-selective receptor-mediated pathway may explain the unique physiologic responses of human airways to LTE4 in vivo.
Cysteinyl leukotrienes (cys-LTs)2 (LTC4, LTD4, LTE4) are potent inflammatory mediators derived from arachidonic acid and generated by mast cells (MCs), eosinophils, basophils, and macrophages (reviewed in Ref. 1). Arachidonic acid is liberated from nuclear membrane phospholipids by a cytosolic phospholipase A2 (2) and converted by 5-lipoxygenase (5-LO) and its molecular partner, 5-LO-activating protein (FLAP), to the unstable intermediate LTA4 at the nuclear envelope (3, 4). LTA4 is then conjugated to reduced glutathione by an integral nuclear membrane protein, leukotriene C4 synthase (LTC4S) (5, 6), forming LTC4. After transport to the extracellular space by multidrug resistance protein-1 (7), LTC4 is converted extracellularly to LTD4 by a γ-glutamyl leukotrienase (8), and then to the terminal product LTE4 by a dipeptidase (9). This rapid conversion ensures that LTC4 and LTD4 are very short-lived in vivo. In contrast, LTE4 is stable, being the only cys-LT detected in biologic fluids and excreted in the urine without further modification (10). Cys-LTs are the most potent known bronchoconstrictors (11, 12), and they also potentiate airway hyperresponsiveness (AHR) to histamine when they are administered by inhalation to human subjects (13). Bronchoalveolar lavage (BAL) fluids collected from allergen-challenged atopic asthmatic individuals contain high levels of cys-LTs (14), and levels of LTE4 are elevated in urine samples from patients during spontaneous asthmatic exacerbations (10). Drugs that block the type 1 receptor for cys-LTs (CysLT1R) (15, 16) or that interfere with cys-LT synthesis (17) are clinically efficacious in asthma. Studies with mice lacking LTC4S and/or cys-LT receptors suggest additional prominent functions for these mediators in adaptive immunity and fibrosis (18–20). Thus, mechanisms that control cys-LT-dependent biologic responses are of considerable pathobiologic and clinical interest in both allergic and nonallergic disease.
CysLT1R and CysLT2R are the two known G protein-coupled receptors (GPCRs) selective for cys-LTs (21, 22). CysLT1R is expressed prominently by smooth muscle and leukocytes (22, 23), while CysLT2R is expressed by cardiac Purkinje cells, endothelium, brain, and leukocytes (21). A third receptor, GPR17, recognizes both LTD4 and uracil nucleotides and is expressed primarily in the brain (24). CysLT1R binds LTD4 with higher affinity than LTC4 (EC50 values for binding of 10–9 m and 10–8 m, respectively) (22), whereas CysLT2R has equal affinity for LTD4 and LTC4 (EC50 of 10–8 m for each) (21). LTE4 is a weak, partial agonist for CysLT1R and CysLT2R, binding each with 1–2-log fold lower affinity than do LTC4 and LTD4 (21, 23). Although it is a modest bronchoconstrictor relative to LTD4 (25), LTE4 nonetheless elicits biologic responses than are distinct from those induced by its precursors. After inhalation by human subjects, LTE4 (but not LTD4) causes significant increases in the numbers of eosinophils, basophils, and MCs in sputum over several hours (25, 26). Humans with aspirin-exacerbated respiratory disease (AERD), a variant of asthma characterized by markedly elevated baseline generation of cys-LTs, exhibit bronchoconstrictor responses to inhaled LTE4 that are disproportionate relative to their responses to histamine (27), LTC4, or LTD4 (28). Prior inhalation of LTE4 by humans with asthma potentiates AHR to histamine; this response can be blocked by pretreatment of the subjects with the cyclooxygenase (COX) inhibitor indomethacin (29). Likewise, LTE4 (but not LTC4 or LTD4) potentiates contraction of guinea pig tracheal rings to histamine in an indomethacin-sensitive fashion (30). Thus, LTE4-induced pulmonary responses in vivo are dissimilar to those caused by LTC4 and LTD4, are not explained by the pharmacology of the established GPCRs for cys-LTs, and may be mediated by induced prostanoids.
MCs are stem cell factor (SCF)-dependent hematopoietic cells that are ubiquitously distributed at interfaces with the external environment (reviewed in Ref. 31, 32) and abound in human airways. MCs trigger exacerbations of asthma through the elaboration of soluble mediators. Among these are especially large quantities of prostaglandin D2 (PGD2), a COX product that is a bronchoconstrictor and chemoattractant for eosinophils, basophils, and Th2 cells. MCs express both CysLT1R and CysLT2R (33, 34), which form heteromeric complexes on these cells (35). Stimulation of primary human MCs derived in vitro from cord blood progenitors (hMCs) with LTD4 potently induces calcium flux (32), extracellular signal-regulated kinase (ERK) phosphorylation, and cytokine generation (36). Based on RNA interference and/or pharmacologic antagonism with MK571, a drug that blocks CysLT1R but not CysLT2R, each of these responses requires CysLT1R. In a model of allergen-induced pulmonary inflammation, LTC4S–/– mice showed a striking deficit in the number of MCs in the tracheal epithelium (20). In a separate study, exogenous LTD4 induced the proliferation of hMCs by causing transactivation of c-Kit, the receptor for SCF, through CysLT1R (37), while CysLT2R counter-regulates these responses (35). Unexpectedly, despite its weak activity at CysLT1R and CysLT2R, LTE4 increased the numbers of MCs arising from liquid culture of cord blood mononuclear cells more potently than LTC4 or LTD4 (37). We now report that LTE4 signals though a distinct, MK571-sensitive pathway independent of CysLT1R and CysLT2R,, thereby linking extracellular LTE4 to peroxisome proliferator-activated receptor γ (PPARγ)-dependent ERK activation, inducible expression of COX-2, and generation of PGD2. These findings support the possible existence of a LTE4-activated GPCR that accounts for the distinct effects of LTE4 in vivo.
EXPERIMENTAL PROCEDURES
Reagents—LTD4, LTE4, PGJ2, GW9662, NS398, MK571, and anti-COX-2 and PPARγ Abs were purchased from Cayman Chemical. Fura-2 AM was from Molecular Probes, and all primers were from SuperArray. The phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 and all phosphospecific Abs were from Cell Signaling. A second PPARγ Ab was from UBI. The siRNA for PPARγ was from Dharmacon, pertussis toxin (PTX) was from Sigma, and PD98059 was from Chemicon.
Cell Culture—The LAD2 line (38) isolated from the bone marrow of a patient with MC leukemia was a kind gift of Dr. Arnold Kirshenbaum (NIH). These cells were cultured in Stem-pro 34™ (Invitrogen) supplemented with 2 mm l-glutamine (Invitrogen), Pen-strep (100 international units/ml) (Invitrogen), and SCF (Endogen) (100 ng/ml). Cell culture medium was hemi-depleted every week with fresh medium and 100 ng/ml SCF. Primary hMCs were derived from cord blood mononuclear cells cultured for 6–9 weeks in RPMI supplemented with SCF, interleukin IL-6, and IL-10 (39).
Calcium Flux—LAD2 cells (0.5–1 × 106/sample) were washed and labeled with fura 2-AM for 30 min at 37 °C. Cells were stimulated with the indicated concentrations of LTC4, LTD4, and LTE4, and changes in intracellular calcium concentration were measured using excitation at 340 and 380 nm in a fluorescence spectrophotometer (Hitachi F-4500) (34). The relative ratios of fluorescence emitted at 510 nm were recorded and displayed as a reflection of intracellular calcium concentration. In some experiments, cells were preincubated with the CysLT1R antagonist MK571 (1 μm) for 5 min before the stimulation.
Flow Cytometry—The expressions of Kit, CysLT1R, CysLT2R, GPR17, and PPARγ in LAD2 cells were determined by flow cytometry. Briefly, LAD2 cells (2 × 105) were washed in fluorescence-activated cell sorting (FACS) buffer (1% bovine serum albumin, 0.2 mm EDTA in phosphate-buffered saline), fixed with 4% paraformaldehyde, and incubated with mouse anti-human IgG1 against Kit (BIOSOURCE International) or with custom-generated Abs against extracellular domains of the human CysLT1R (RB34) (35) and CysLT2R (RB19) (Orbigen). In some experiments, polyclonal Abs against the C termini of human CysLT1R and CysLT2R (Cayman) were used. For experiments with the latter Abs, as well as those used to detect intracellular PPARγ, the cells were permeabilized with 0.5% saponin before staining, followed by a fluorescein isothiocyanate-conjugated secondary Ab for another 30 min. Staining for GPR17 was done using a polyclonal Ab raised against the extracellular N terminus (Novus) with and without permeabilization. Nonspecific rabbit IgG and mouse IgG1 (BioSource International) were used as respective negative controls. Cells were washed with FACS buffer three times, and flow cytometric analyses were performed with a Becton-Dickinson FACScan flow cytometer.
Real-time Quantitative PCR—The expressions of CysLT1R, CysLT2R, macrophage inflammatory protein-1β (MIP-1β), MCP-1, IL-5, IL-8, COX-1, COX-2, phospholipase A2 (PLA2) (groups IIA, IVA, V, and X), hematopoietic PGD2 synthase (PGDS), and tumor necrosis factor α (TNF-α) mRNAs were determined with real-time PCR performed on an ABI PRISM 7700 Sequence detection system (Applied Biosystems). LAD2 cells were growth factor-starved overnight and stimulated with LTD4 or LTE4 (100 nm) or with medium alone for 2 h at 37 °C. RNA was isolated with an RNAeasy minikit (Qiagen) and was treated with RNase-free DNase (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 1 μgof RNA with Superscript II RNase H-RT (Invitrogen). Reverse transcription (RT) was performed using TaqMan RT reagents. All primers and FAM-labeled PCR mix were purchased from Superarray.
Short Hairpin RNA (shRNA) and Small Interfering RNA (siRNA) Knockdowns—shRNA constructs targeting human CysLT1R and CysLT2R were purchased from Open Biosystems. The constructs were cloned into a lentiviral vector (pLKo1, Open Biosystems) and used to generate infectious particles with a lentiviral packaging mix (Virapower, Invitrogen) according to the manufacturer's protocol. The transfections were carried out as described previously (35). FACs analysis was used to confirm the knockdowns. siRNA against PPARγ and scrambled double-stranded RNA controls were purchased from Dharmacon in the form of a SMART pool. Cells were transfected with 50 nm PPARγ and scrambled siRNAs using Lipofectamine according to the manufacturer's instructions. At 48 h, knockdowns were confirmed by Western blotting, and the cells were used for the indicated assays.
Cell Activation—LAD2 cells and primary hMCs either were stimulated with the indicated concentrations of LTD4 or LTE4 or were passively sensitized with human myeloma IgE (2 μg/ml; Chemicon) overnight and stimulated with rabbit anti-human anti-IgE (Chemicon, 1 μg/ml), SCF (100 ng/ml), PGJ2 (20 μg/ml), or rosiglitazone (10 μm), a PPARγ agonist. To determine the contribution of various signaling events in agonist-mediated responses, cells were stimulated after preincubation with PTX (100 ng/ml) for 18 h; with the PPARγ antagonist GW9662 (10 μm) for 1 h; or with MK571 (1 μm), the mitogen-activated protein kinase kinase (MEK) inhibitor PD98058 (50 μm), the PI3K inhibitor LY294002 (10 μm), the cytosolic PLA2 (cPLA2) inhibitor Shinogi 1 (5 μm), or the COX-2 inhibitor NS398 (10 μm) for 30 min. Cells were stimulated with the agonists for 15 min for ERK phosphorylation, 2 h for PCR analysis, 6 h for the measurement of cytokine and PGD2 generation, and 18 h for the PPARγ ligand-binding domain (LBD) assay (40). The concentration of MIP-1β was measured by an ELISA (Endogen). PGD2 was detected using a PGD2-methoxylamine hydrochloride (PGD2-MOX) assay (Cayman). The PGD2 values detected with this assay were similar to those identified in the supernatants of cys-LT-stimulated primary hMCs and LAD2 cells by metabolite separation and analysis by reversed-phase HPLC and electrospray ionization-mass spectrometry (LC-MS) (40).
Cell Lysates and Western Blotting—After stimulation with the respective agonists, LAD2 cells and primary hMCs (0.5 × 106) were lysed with lysis buffer (BD Bioscience) supplemented with protease inhibitor mixture (Roche Applied Science) and sodium vanadate (1 mm). Lysates were subjected to 4–12% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were incubated with Abs against phospho- and total ERK, MEK, 90 kDa ribosomal s6 kinase (p90RSK), and cyclic AMP-regulated-binding protein (CREB) (Cell Signaling Technologies) in 1× phosphate-buffered saline, 5% dry milk, 0.1% Tween-20 (1:1000) overnight at 4 °C on shaker, and then with secondary Ab (peroxidase-conjugated anti-rabbit or anti-mouse). Bands were visualized using enhanced chemiluminescence (Pierce).
PPARγ LBD Assay—Bovine aortic endothelial cells, and CHO cells stably transfected with human CysLT1R or CysLT2R were plated in 24-well plates and transiently transfected in 1% delipidated plasma (DLP)/DMEM per the manufacturer's instructions (Fugene HD, Roche Applied Science). Briefly, cells were co-transfected with constructs for the human PPARγ-LBD GAL4 fusion, the GAL4-responsive luciferase reporter pUASX4TK-luc, and β-galactosidase. Cells were stimulated with the indicated reagents for 18–24 h before the PPARγ LBD-GAL4 assays were performed. Luciferase counts, normalized to β-galactosidase activity, were obtained using luciferase substrates (BD Pharmingen); chlorophenol red-β-d-galactopyranoside was used for β-galactosidase activity assays (Roche Diagnostics) (41).
Cell Proliferation—Mitogenic assays were performed in triplicate on cells suspended in fresh medium at a concentration of 0.5 × 106/ml with or without LTD4 and LTE4 (0.01–0.1 μm) in the absence of SCF. In some experiments, MK571 (1 μm) or GW9662 (10 μm) was added at the same time as the mitogens. At 48 h, the cells were pulsed overnight with [3H]thymidine (Amersham Biosciences), and counts were analyzed by β-counting. The radioactivity incorporated was measured in triplicate, and the results are expressed as mean ± S.D.
Statistics—Data are expressed as mean ± S.D. from at least three experiments except where otherwise indicated. Data were converted to a percentage of control for each experiment where indicated. The significance was determined with the Student's t test.
RESULTS
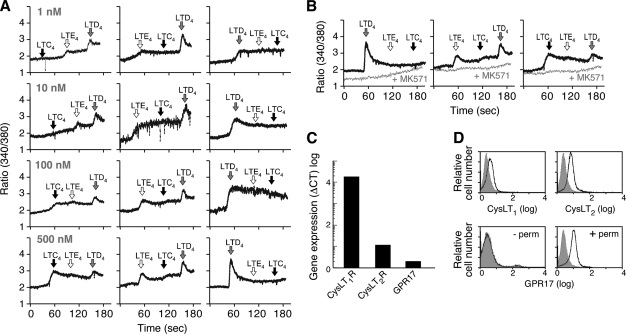
Rank Order of cys-LTs for Inducing Calcium Flux in LAD2 Cells—To determine the potency of LTE4 for calcium flux relative to the other cys-LTs, we stimulated Fura-2-loaded LAD2 cells with various doses of each cys-LT and performed cross-desensitizations. LTD4 was the most potent agonist among the cys-LTs for eliciting calcium flux and completely desensitized the LAD2 cells to the calcium fluxes induced by both LTC4 and LTE4 (Fig. 1A). LTE4 caused calcium flux at doses as low as 1 nm that was not attenuated by prior stimulation of the cells with an equal amount of LTC4. LTC4 did not induce a calcium flux at concentrations below 100 nm. LTE4 partly desensitized LAD2 cells to LTD4 and completely desensitized these cells to LTC4 (Fig. 1B). Regardless of the cys-LT used to stimulate the LAD2 cells, the calcium responses were totally blocked by pretreatment of the cells with MK571 (Fig. 1B), which competitively antagonizes CysLT1R but not CysLT2R. Thus, although LAD2 cells express CysLT2R mRNA (Fig. 1C) and protein (Fig. 1D), all cys-LT-induced calcium flux in these cells is mediated by MK571-sensitive receptors. GPR17 was detected intracellularly but not on the surfaces of the LAD2 cells.
FIGURE 1.
cys-LT receptor expression and calcium signaling by LTD4 and LTE4 in LAD2 cells. A, dose-dependent effects of LTC4, LTD4, and LTE4 on the accumulation of intracellular calcium in LAD2 cells. LAD2 cells were loaded with Fura-2-AM and stimulated with the indicated concentrations of LTs in various orders. B, effect of treatment with MK571 (1 μm, 5 min) on calcium flux by Fura-2-AM-loaded LAD2 cells stimulated with the indicated LTs (500 nm each). C, real-time PCR analysis of CysLT1R, CysLT2R, and GPR17 transcripts expressed by LAD2 cells. D, flow cytometry analysis of CysLT1R, CysLT2R, and GPR17 proteins. CysLT1R and CysLT2R were detected after permeabilization of LAD2 cells and staining with anti-C terminus Abs. GPR17 staining was performed with an anti-N terminus Ab both with (+perm) and without (–perm) permeabilization. Shaded curves are staining with nonspecific rabbit IgG. Results depicted are from single experiments, representative of at least three performed for each assay.
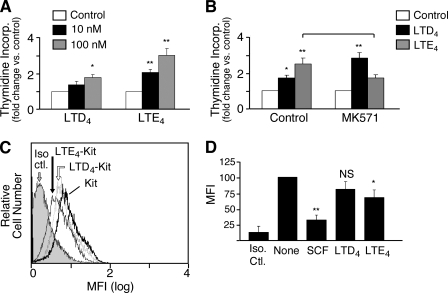
cys-LT-mediated Proliferation and Kit Internalization—We compared the effects of LTD4 with those of LTE4 for inducing proliferation of LAD2 cells. Unlike primary hMCs, LAD2 cells do not depend on exogenous SCF for their survival (38), and they exhibit constitutive phosphorylation of Kit (not shown). We therefore tested the ability of each cys-LT to stimulate thymidine incorporation in the absence of SCF. As anticipated, both LTD4 and LTE4 caused dose-dependent increments in thymidine incorporation when provided to LAD2 cells. The proliferation caused by LTE4 was greater than that induced by LTD4 at both 10 and 100 nm (Fig. 2A). Whereas LTD4-mediated proliferation was slightly potentiated by MK571, LTE4-mediated cell proliferation was blocked (Fig. 2B). Neither basal nor cys-LT-mediated proliferation of LAD2 cells was sensitive to treatment with the Kit inhibitor STI571 (not shown). Nonetheless, LTE4 caused the internalization of Kit, a signature event of receptor tyrosine kinase transactivation (42) to a greater extent than did LTD4 (Fig. 2, C and D).
FIGURE 2.
Effect of cys-LTs on proliferation and Kit internalization in LAD2 cells. A, dose-dependent effect of LTD4 and LTE4 on thymidine incorporation by LAD2 cells stimulated for 48 h in the absence of SCF. B, effect of MK571 added 30 min before the addition of the cys-LTs on cell proliferation. Results are expressed as the mean ± S.D. of triplicate samples in a single experiment representative of the three performed. C, flow cytometric analysis of surface Kit expression by LAD2 cells stimulated with SCF (as a positive control for internalization), LTD4, or LTE4 for 1 h before staining with an Ab specific for Kit receptor or an isotype-matched control. MFI, mean fluorescence intensity; Iso ctl., isotype control (mouse IgG1). D, effect of cys-LTs on Kit surface staining expressed as net MFI. Data represent mean ± S.D. from the three experiments performed. * indicates p < 0.05, relative to the control, and ** reflects p < 0.01 compared with the LTD4-treated samples at the same doses.
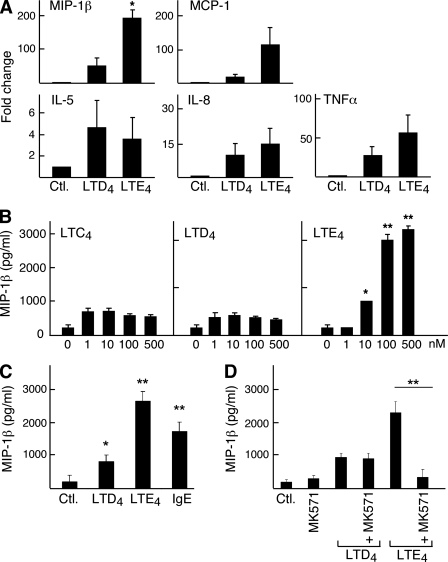
Induction of Chemokines and Cytokines by cys-LTs—LTC4 and LTD4 both induce the production of proinflammatory cytokines and chemokines by primary hMCs. We sought to determine whether LTE4 also induced cytokine generation by LAD2 cells, and if so, whether it was equivalent in potency to LTD4. Real-time PCR analysis showed that cys-LT stimulation of LAD2 cells induced the expression of MIP-1β, IL-5, IL-8, TNF-α, and CCL-2 (MCP-1) mRNA transcripts (Fig. 3A). LTE4 tended to be more potent than LTD4 for inducing the expression of each transcript with the exception of IL-5. Consistent with the mRNA data, LTE4 induced MIP-1β generation in a dose-dependent manner (Fig. 3B). The quantity of MIP-1β generated by LAD2 cells stimulated with LTE4 exceeded the amount produced in response to cross-linkage of the high affinity Fc receptor for IgE (FcεRI) (Fig. 3C). LTE4-mediated MIP-1β production was totally blocked by pretreatment of the cells with MK571 (Fig. 3D).
FIGURE 3.
Cytokine mRNA induction and MIP-1β generation by LAD2 cells stimulated with cys-LTs. A, real-time PCR showing relative levels of MIP-1β, MCP-1, IL-5, IL-8, and TNFα transcript expression by LAD2 cells stimulated with 100 nm LTD4 or LTE4 for 2 h. Data are mean ± S.E. from three experiments. B, dose-dependent effect of LTE4 on MIP-1β secretion by LAD2 cells stimulated for 6 h with the indicated concentrations of cys-LTs. Results are mean ± S.E. from three experiments. C, MIP-1β concentrations were measured in supernatants collected from cells after 6 h of stimulation with LTD4 (100 nm), LTE4 (100 nm), or anti-IgE (1 μg/ml) as a positive control. D, effect of pretreatment with MK571 (1 μm) on MIP-1β generation by cells stimulated with LTD4 (100 nm), or LTE4 (100 nm) for 6 h. Results are expressed as the mean ± S.D. from the three experiments performed. * and ** indicate p < 0.05 and <0.01, respectively, relative to the control in B and C and relative to the samples without MK571 in D.
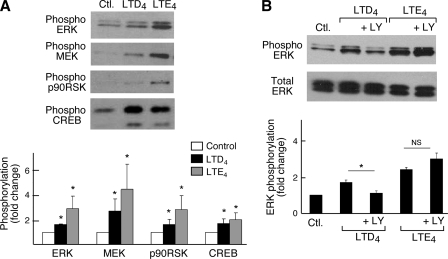
LTE4-induced Changes in Phosphorylation of Signaling Intermediates—LTD4-mediated proliferation and cytokine generation by primary hMCs both require the phosphorylation of ERK (36, 37). Because LTE4 potently induced cytokine generation and proliferation, we analyzed the ability of LTD4 and LTE4 to induce phosphorylation of various signaling intermediates in the ERK pathway. Stimulation of LAD2 cells with LTD4 or LTE4 for 15 min led to the phosphorylation of MEK, ERK, p90RSK, and CREB. LTE4 was the most potent stimulus for phosphorylation of each of these proteins (Fig. 4A). Neither JNK nor p38 MAPKs were phosphorylated in response to either cys-LT (data not shown). LTD4-induced ERK phosphorylation was attenuated by pretreatment of the LAD2 cells with LY294002, a PI3K inhibitor. In contrast, LTE4-enhanced ERK activation was insensitive to PI3K inhibition (Fig. 4B).
FIGURE 4.
Phosphorylation of signaling intermediates by LAD2 cells in response to cys-LTs. A, SDS-PAGE immunoblotting was performed on cell lysates obtained after 15 min of cell stimulation with LTD4 (100 nm) and LTE4 (100 nm), using Abs specific for total and phosphorylated (phospho) ERK, MEK, p90RSK, and CREB (top). Representative blots are from a single experiment of three performed. The bottom panel indicates the quantitative densitometry where phosphorylation is the measure of phosphorylated protein compared with the total protein and is expressed as fold change compared with control, where the control is set to 1. Data are expressed as mean ± S.D. from three experiments. B, effect of treatment of the cells with the PI3K inhibitor LY294002 (10 μm)(LY) for 30 min on ERK phosphorylation in response to stimulation with 100 nm cys-LTs. The bottom panel represents quantitative densitometry of ERK phosphorylation compared with total ERK from three separate experiments. * indicates p < 0.05 relative to the LTD4-stimulated sample not treated with LY294002.
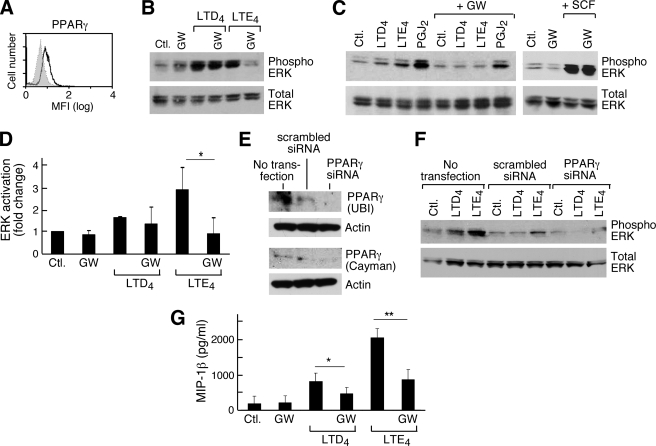
Involvement of PPARγ in LTE4-mediated ERK Activation—PPARγ is a member of the nuclear hormone receptor family involved in the transcriptional regulation of adipogenesis, insulin sensitivity, lipid metabolism, and inflammation (43, 44). PPARγ is activated by certain lipid mediators, including intracellular activators formed in response to transmembrane stimuli (45). In some cell types, exogenous PPARγ activators stimulate MEK and ERK phosphorylation (46–48), which can be dependent (46) or independent (47–49) of PPARγ itself. Both the thiazolidinedione PPARγ agonist rosiglitazone and lysophosphatidic acid, a natural PPARγ activator, potentiated the proliferation of primary hMCs (50). We thus tested whether LTE4-mediated ERK activation involved PPARγ. LAD2 cells expressed PPARγ protein (Fig. 5A). GW9662, an antagonist of PPARγ, modestly attenuated LTD4-mediated ERK phosphorylation (as shown for one experiment, Fig. 5B) but consistently blocked LTE4-mediated ERK phosphorylation (Fig. 5, B–D). GW9662 did not alter SCF-induced ERK phosphorylation. Treatment of LAD2 cells with a PPARγ-specific siRNA for 48 h substantially knocked down the expression of the protein (Fig. 5E) and interfered with LTE4-induced ERK phosphorylation (Fig. 5F). The natural PPARγ activator, 15-deoxy-PGJ2 (15-d-PGJ2), also stimulated ERK phosphorylation in LAD2 cells (Fig. 5C), but rosiglitazone neither induced ERK activation nor potentiated the ERK activation or PGD2 in response to LTC4 or LTD4 (n = 2, data not shown). ERK activation was not attenuated by pretreating the cells with the cPLA2 inhibitor Shinogi 1 (n = 2, not shown). GW9662 strongly interfered with MIP-1β generation by LAD2 cells (Fig. 5G) and by primary hMCs (not shown).
FIGURE 5.
Involvement of PPARγ in cys-LT-induced responses in LAD2 cells. A, flow cytometric analysis on permeabilized LAD2 cells using an Ab specific for PPARγ or an isotype-matched control (shaded curve). Data in a second experiment were identical. B and C, ERK phosphorylation by LAD2 cells in response to stimulation with LTD4 (100 nm), LTE4 (100 nm), PGJ2 (20 μg/ml), or SCF (100 ng/ml) for 15 min. The samples were preincubated in the presence or absence of GW9662 (10 μm) (GW) for 1 h before stimulation with the indicated agonists. Representative blots from the three experiments are shown. D, densitometric analysis (mean ± S.D. of three separate experiments) showing the effects of GW9662. E, siRNA-mediated knockdown of PPARγ. Immunoblotting was performed using two different Abs from the indicated sources. F, effect of the PPARγ knockdown or treatment with scrambled siRNA control assessed by phosphorylation of ERK in response to the indicated agonists for 15 min. Data are from a single experiment representative of three. G, MIP-1β concentrations were measured in supernatants collected after cells were stimulated for 6 h with LTD4 (100 nm) or LTE4 (100 nm). Some of the cells were pretreated with GW9662 (10 μm) for 1 h. Data are the mean ± S.D. of three independent experiments. * and ** indicate p < 0.05 and <0.01, respectively.
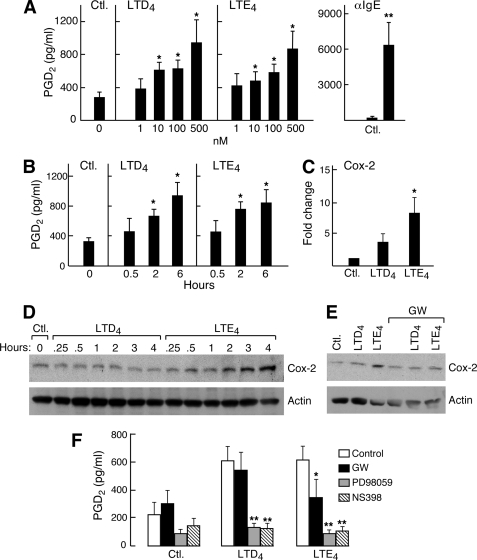
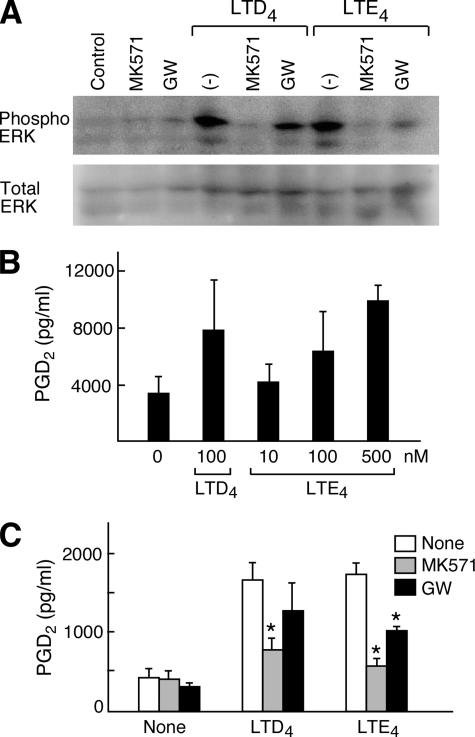
Induction of COX-2 Expression and PPARγ-dependent PGD2 Production by LTE4—LTE4-mediated potentiation of AHR depends on secondary generation of prostanoids, based on its sensitivity to blockade by the COX inhibitor indomethacin (28, 29). The major prostanoid generated by MCs is PGD2, a strong potentiator of hyperresponsiveness to methacholine and histamine (51). To determine whether LTE4 caused LAD2 cells to generate PGD2, we stimulated the cells with cys-LTs for various intervals of time. LTE4 induced the generation of PGD2; the effect was significant at 10 nm LTE4 and marked at 500 nm (Fig. 6A). This response was time-dependent and peaked at 6 h (Fig. 6B). LTE4 was equivalent in potency to LTD4 for this response. The production of PGD2 induced by LTE4 was associated with increased expression of COX-2 mRNA (which peaked at 2 h) (Fig. 6C) and protein (which peaked at 4 h) (Fig. 6D). LTE4 exceeded the potency of LTD4 for these responses. Neither LTD4 nor LTE4 up-regulated the expression of groups IIA, IV, V, or X PLA2, COX-1, or PGDS (n = 3, not shown). LTE4-mediated PGD2 generation (not shown) and COX-2 induction (Fig. 6E) were blocked by treatment of the cells with GW9662. MK571 and PTX (not shown) also blocked LTE4-mediated PGD2 generation. In agreement with the pharmacological inhibition data, knockdown of PPARγ also attenuated LTE4-mediated production of PGD2 in two separate experiments (54 and 67% inhibition of PGD2 production compared with cells treated with a control siRNA, data not shown) without altering the baseline. Treatment of the LAD2 cells with NS-398, a COX-2-selective inhibitor; PD98058, an inhibitor of MEK-ERK signaling; or Shinogi-1 abrogated the production of PGD2 occurring in response to LTE4 and also abrogated basal PGD2 secretion (not shown). Primary hMCs, in which LTE4 also caused GW9662-sensitive ERK activation (Fig. 7A) also exhibited dose-dependent PGD2 generation in response to LTE4 (Fig. 7B) that was both GW9662- and MK571-sensitive (Fig. 7C); a similar trend was observed for MIP-1β production (n = 2, data not shown). LTD4 and LTE4 were equipotent for eliciting these responses from primary hMCs.
FIGURE 6.
PGD2 production and up-regulation of COX-2 expression by LAD2 cells in response to cys-LTs and the involvement of PPARγ in mediating these effects. A, dose response of cys-LT-mediated PGD2 generation in LAD2 cells stimulated with the indicated concentrations of cys-LTs for 6 h. B, time course of PGD2 generation by LAD2 cells stimulated for the indicated intervals with LTD4 (500 nm) or LTE4 (500 nm). C, real-time PCR showing relative levels of COX-2 transcript expression by LAD2 cells stimulated with 100 nm LTD4 or LTE4 for 2 h. Data in A–D are the mean ± S.E. from three experiments each. D, time course of COX-2 protein induction in cells stimulated with LTD4 (100 nm) and LTE4 (100 nm) for the indicated periods of time. Data are from a single experiment representative of the three performed. E, effect of GW9662 (10 μm, 1 h) on COX-2 protein up-regulation in response to LTD4 and LTE4 (100 nm for 4 h each,). Data are from a single experiment representative of three performed. F, effect of pretreatment with GW9662 (10 μm, 1h), PD98059 (50 μm, 30 min), and NS398 (10 μm, 30 min) on LTE4-induced generation of PGD2 (measured 6 h after stimulation) by LAD2 cells. Data depicted are the mean ± S.D. of three independent experiments. PGD2 was quantitated with a PGD2-MOX assay.
FIGURE 7.
Effect of LTD4 and LTE4 on PPARγ-dependent ERK activation and PGD2 generation by primary hMCs. A, cord blood-derived hMCs (6-week-old) were stimulated for 10 min with LTD4 or LTE4 (500 nm) in the absence or presence of GW9662 (10 μm) or MK571 (1 μm). Results are from a single experiment representative of three separate experiments performed. B, PGD2 generation induced by stimulation of primary hMCs with LTD4 or LTE4 at the indicated concentrations. Results are the mean ± ½ range for two experiments. C, effects of GW9662 (10 μm) or MK571 (1 μm) on cys-LT-induced PGD2 generation by primary hMCs. Cells were stimulated with 100 nm of the indicated cys-LT for 6 h. Results were measured with the PGD2-MOX assay and are the mean ± S.E. of three independent experiments. * indicates p < 0.05.
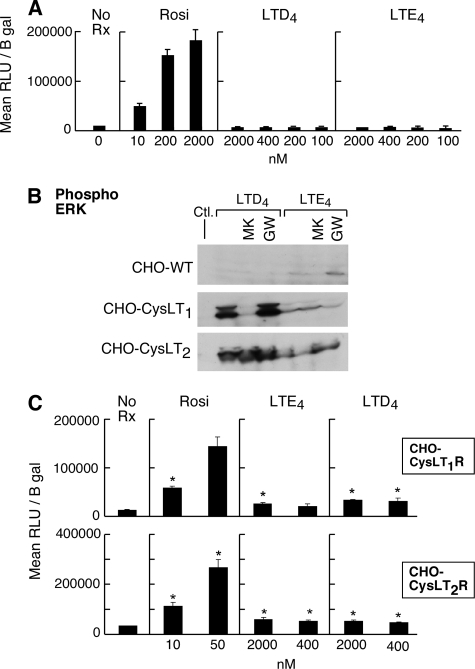
Independence of LTE4 Effects from Direct PPARγ Activation—To determine whether LTE4 directly activated PPARγ, we stimulated bovine endothelial cells expressing a PPARγ LBD-driven luciferase reporter construct (41) with LTD4, LTE4, or rosiglitazone as a positive control. In this assay, luciferase activity measured as a colorimetric readout indicates PPARγ activation. Neither LTD4 nor LTE4 activated the luciferase promoter in this cell type, whereas rosiglitazone did activate PPARγ (Fig. 8A). Neither LTD4 nor LTE4 potentiated rosiglitazone-induced PPARγ activation (not shown). To determine if LTE4-mediated PPARγ activation (as evidenced by the LBD assay and GW9662-sensiitve ERK activation) depended on expression of known cys-LT-specific GPCRs, we compared cys-LT-induced ERK activation in CHO cells (which lack endogenous cys-LT receptors) with and without stable expression of CysLT1R or CysLT2R. Cys-LTs failed to elicit ERK phosphorylation in mock-transfected CHO cells. Heterologous expression of either CysLT1R or CysLT2R conferred ERK phosphorylation to both ligands, but LTD4 was far more potent than LTE4 at both receptors. MK571 blocked the strong LTD4-mediated activation of ERK in the CysLT1R transfectants, but not in the CysLT2R transfectants. GW9662 treatment attenuated the weak LTE4-induced activation of ERK in the CysLT1R transfectants, but did not alter LTD4-mediated ERK activation (n = 3, as shown for one experiment, Fig. 8B). Both LTD4 and LTE4 weakly induced activation of the PPARγ LBD luciferase construct when the cells expressed either CysLT1R or CysLT2R (Fig. 8C). Attempts to transfect LAD2 cells with the PPARγ LBD construct were unsuccessful.
FIGURE 8.
Lack of direct stimulation of PPARγ by LTE4 in heterologous cell systems. A, PPARγ-specific LBD assay in bovine endothelial cells in response to stimulation with rosiglitazone (rosi), LTD4, or LTE4. Results are expressed as relative light units corrected for perβ-galactosidase activity (RLU/β-gal). B, phosphorylation of ERK by CHO cells with or without transduced expression of CysLT1R or CysLT2R in response to the indicated cys-LTs, with or without MK571 or GW9662. C, activation of PPARγ specific LBD in CHO cells expressing CysLT1R or CysLT2R. Results in A, B, and C are each from single experiments repeated a minimum of twice. A includes triplicate samples. * indicates p < 0.05 relative to control.
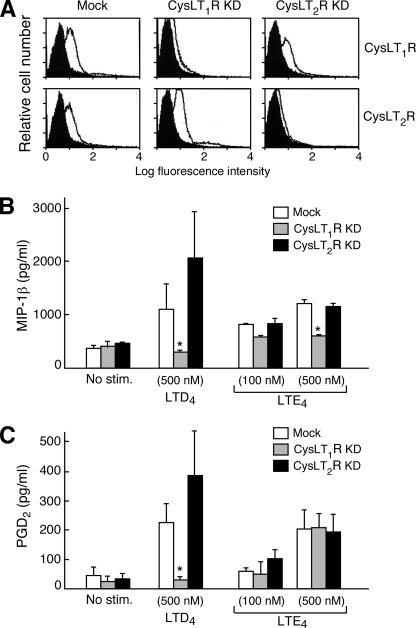
Effect of CysLT1R and CysLT2R Knockdowns on Activation of LAD2 Cells by LTE4—To determine whether LTE4-induced signaling depended on the conventional cys-LT responsive GPCRs, we knocked down the expression of CysLT1R and CysLT2R on LAD2 cells using sequence-specific shRNA. As reported previously (35), the knockdowns were highly efficacious and selective (Fig. 9A). Knockdown of CysLT1R abrogated MIP-1β generation (Fig. 9B) and PGD2 production (Fig. 9C) in response to LTD4, whereas CysLT2R knockdown tended to potentiate these responses. Strikingly, although LTE4-mediated MIP-1β generation was attenuated by CysLT1R knockdown (Fig. 9B), PGD2 generation was unaltered (Fig. 9C), and CysLT2R knockdown had no effect on either response. Lentiviral transfection markedly attenuated proliferation in all groups (not shown).
FIGURE 9.
Effect of shRNA-mediated knockdown of CysLT1R and CysLT2R on LTE4-mediated PGD2 generation by LAD2 cells. A, FACS analysis of nonpermeabilized LAD2 cells showing the effect of the knockdowns after 48 h of treatment with lentivirus containing shRNA directed to the indicated receptors, or with empty virus (Mock). Results are from a single experiment representative of the three separate experiments performed. B, MIP-1β generation by LAD2 cells stimulated for 6 h with the indicated concentrations of LTE4 or LTD4 after treatment with CysLT1R- and CysLT2R-specific shRNA constructs packaged in lentivirus, or with control vector. C, PGD2 generation by LAD2 cells treated with the same lentiviral vectors. Results in B and C are expressed as the mean ± S.E. of the same three experiments, including the one depicted in A. * indicates p < 0.05.
DISCUSSION
This study establishes that LTE4, the weakest agonist of the known cys-LT receptors, is unexpectedly potent for inducing proliferative signaling and transcriptional responses from MCs. The potency of LTE4 reflects apparent cooperation between an MK571-sensitive GPCR (potentially other than CysLT1R) and PPARγ-dependent ERK signaling and up-regulation of COX-2. These events result in induction of PGD2 synthesis and chemokine generation. These findings may explain the unique LTE4-mediated biologic responses previously described in human (29) and guinea pig airways (30) that depend on the secondary generation of COX products. The findings also likely explain our previous observation that LTE4 exceeded the potency of LTC4 and LTD4 for augmenting the development of cord blood-derived hMCs in vitro (37).
LAD2 cells, a well-differentiated human MC sarcoma line (38), express both CysLT1R and CysLT2R (35) (Fig. 1, C and D) and thus provided a convenient system for studying integrated cys-LT-mediated signaling. LTD4 far exceeded the potency of LTC4 for calcium flux in LAD2 cells, as anticipated for a CysLT1R-dependent event (Fig. 1A). Unexpectedly, however, LTE4 ranked between LTD4 and LTC4 for inducing calcium flux, a profile differing from any known single cys-LT receptor response. CHO cells expressing CysLT1R or CysLT2R flux calcium strongly in response to LTC4 and LTD4, but negligibly in response to LTE4 (not shown). Although MK571 does not block CysLT2R-mediated ligand binding or signaling, it does block other CysLT1R homologues, including several purinergic GPCRs (52) and the recently de-orphanized GPR17 (24). The calcium flux data support the possible presence of an MK571-sensitive LTE4-responsive GPCR that is not CysLT1R, as we had also proposed in our previous study (37). This receptor is unlikely to be GPR17, which we did not detect on the LAD2 cell surface (Fig. 1D), and which fails to respond to LTE4 when expressed in CHO cells.3
Cys-LTs induce proliferation and chemokine production by primary hMCs. While LTE4 exceeds the potencies of LTC4 and LTD4 as an accessory growth factor for primary cord blood-derived hMCs (37), we had not previously determined whether LTE4 could induce chemokine generation. LTE4 was ∼2-fold as potent as LTD4 for inducing thymidine incorporation by LAD2 cells (Fig. 2) and for inducing internalization of Kit (Fig. 2, C and D), a likely reflection of transactivation. The pattern for MIP-1β generation and transcript induction was similar, with LTE4 being more potent than LTD4 for LAD2 cells (and equipotent for these responses in hMCs). Each response to LTE4 was blocked by MK571, although, curiously, the weaker responses to LTD4 were resistant in LAD2 cells (but not primary hMCs), again indicating a likely alternate target of MK571 in LAD2 cells. LTD4-mediated proliferation and chemokine generation require CysLT1R-dependent phosphorylation of ERK, which is negatively regulated by CysLT2R (35). Although both LTD4 and LTE4 caused ERK phosphorylation, the LTE4-induced response differed strikingly from the LTD4-mediated response in that it was resistant to PI3K inhibition by LY294002. This difference prompted us to seek a mechanism for LTE4-induced ERK activation through a signaling pathway different from classical LTD4-CysLT1R-regulated responses, and to use a molecular approach to determine the GPCR(s) required.
PPARγ, a ligand-activated nuclear receptor, senses dietary lipids and endogenous lipid mediators (44, 53). Synthetic and natural PPARγ activators can activate ERK and cause proliferation of neuronal stem cells (49), liver tumors (48, 50), and MCs (51). Based on inhibition by the PPARγ antagonist, GW9662, PPARγ was involved in LTE4-induced ERK activation and MIP-1β generation by both LAD2 cells and in primary hMCs. The effect was stimulus-specific, as GW9662 failed to alter ERK activation in response to SCF. GW9662-mediated attenuation of ERK phosphorylation was not due to an off-target effect, because PPARγ knockdown also blocked LTE4-mediated signaling. While the natural PPARγ agonist 15-Δ-PGJ2 mirrored the ability of LTE4 to phosphorylate ERK, rosiglitazone did not. This discrepancy could reflect that fact that thiazolidinedione drugs do not induce recruitment of the same co-activators to the PPARγ signaling complex as do natural activators, which results in divergent functional events (54). As was the case for calcium flux, LTE4 failed to stimulate ERK potently in CHO cells transfected with CysLT1R or CysLT2R (Fig. 8) and did not stimulate the PPARγ LBD in CHO or in bovine endothelial cells. Thus the PPARγ-dependent effects of LTE4 are cell type-specific and indirect, potentially reflecting the secondary generation of an intracellular activator. The putative activator is not an arachidonic acid metabolite liberated by cPLA2 (as reported for PPARγ-dependent responses of bronchial epithelial cells to EGF, Ref. 45) because Shinogi 1 failed to alter ERK activation. Other PLA2s could be responsible for releasing arachidonic acid to generate an endogenous PPARγ activator. Because we were unable to transfect LAD2 cells with the LBD construct, we could not directly assess whether LTE4 activated PPARγ in MCs.
In addition to its role in ERK activation, PPARγ acts as a transcription factor for genes containing PPAR response elements in their promoters, including COX-2 (45, 55). LTE4-dependent potentiation of contractility of guinea pig tracheal rings and of AHR to histamine in humans with asthma can be blocked by indomethacin (13, 30), indicating that some of the biologic functions of LTE4 in the airway may be due to induced secretion of COX products. PGD2, the major COX product of MCs, is a direct bronchoconstrictor (56), a potentiator of AHR (51), and a selective chemoattractant for eosinophils, basophils, and Th2 cells (57). In our study, LTE4 stimulated the production of PGD2 by a COX-2-dependent mechanism, again requiring PPARγ based on both pharmacologic and molecular evidence. The fact that this response could also be blocked by MK571 and by PTX reflected a GPCR requirement. It seems plausible that the induction of COX-2 expression and the subsequent generation of PGD2 may account in part for the ability of LTE4 to recruit eosinophils and basophils to the bronchial mucosa on direct instillation (25). It may also help to explain the fact that LTE4 potentiates AHR in an indomethacin-sensitive fashion.
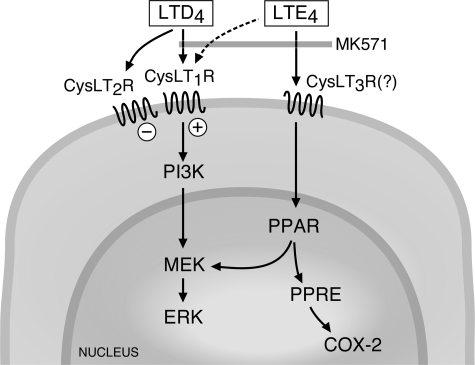
An unexpected finding in our study is the potency of LTE4 as a ligand for signaling events in MCs given its negligible effects at CysLT1R and CysLT2R. Indeed, knockdown of CysLT1R abrogated LTD4-induced generation of both MIP-1β and PGD2, but only partly blocked LTE4-dependent MIP-1β production and left LTE4-mediated PGD2 generation unaffected (Fig. 9, B and C). Moreover, LTE4-induced responses were unaffected by CysLT2R knockdown, which potentiated the responses to LTD4. Thus while certain LTE4 responses may involve its weak agonistic effect at CysLT1R, they are distinct from the balanced positive and negative pathways induced by LTD4 through the CysLT1R/CysLT2R heterodimer (35). Interestingly, LTE4 responses were almost completely MK571-sensitive even in the face of a 90% knockdown of CysLT1R(n = 2, not shown). The remaining ∼200 orphan GPCRs may include an MK571-sensitive LTE4-selective receptor that induces the formation of an endogenous PPARγ ligand, accounting for the observed induction of both chemokines and COX-2-dependent PGD2 (Fig. 10).
FIGURE 10.
Hypothetical mechanism(s) responsible for PPARγ-dependent ERK activation and PGD2 generation by MCs. LTE4-mediated responses are MK571-sensitive and may involve both CysLT1R and an unidentified GPCR, whereas LTD4 responses are regulated by respectively positive and negative signals induced through the CysLT1R/CysLT2R heterodimer. PPRE, PPAR response element.
This study identifies a novel pathway that mediates LTE4-induced signaling in MCs distinct from conventional CysLT1R-dependent responses to LTD4, linking an MK571-sensitive GPCR to ERK and PPARγ. The rapid successive conversion of cys-LTs to LTE4 in vivo limits the duration of direct contractile signaling at the microvasculature and airway smooth muscle, but ensures a comparative abundance of LTE4 in the extracellular space. LTE4-triggered signaling induces the expression of COX-2 and chemokine genes that are intimately associated with inflammatory responses. These findings potentially link several features of AERD, in which a marked abundance of LTE4 (58) is associated with tissue eosinophilia, MC hyperplasia (59), overexpression of both CysLT1R (60), and LTC4S (61), and selective AHR to LTE4 (27). COX-2 protein expression in the bronchial mucosa of patients with AERD is up-regulated in MCs (62) but not other resident cell types, which could reflect a “signature” of the response of MCs to LTE4 and a cause of the elevated PGD2 generation in this syndrome (58). There may be a hierarchical relationship between the two major classes of inflammatory eicosanoids in asthma, explaining previously observed functions unique to LTE4.
Acknowledgments
We thank Professor Robert C. Murphy (National Jewish Medical and Research Center, Denver, CO) for performing the mass spectrometry analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants AI-48802, AI-52353, AI-31599, HL-36110, and EB-00768. This work was also supported by grants from the Charles Dana Foundation, and the Vinik Family Fund for Research in Allergic Diseases. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: cys-LT, cysteinyl leukotriene; Ab, antibody; 5-LO, 5 lipoxygenase; AERD, aspirin-exacerbated respiratory disease; AHR, airway hyper-responsiveness; BAL, bronchoalveolar lavage; COX, cyclooxygenase; CREB, cyclic AMP-regulated-binding protein; CysLT1R, type 1 receptor for cys-LTs; CysLT2R, type 2 receptor for cys-LTs; ERK, extracellular signal-regulated kinase; FACS, fluorescence-activated cell sorting; FcεRI, high-affinity Fc receptor for IgE; FLAP, 5-lipoxygenase activating protein; GPCR, G protein-coupled receptor; hMC, cord blood-derived human MC; IL, interleukin; LBD, ligand binding domain; LC-MS, liquid chromatography-mass spectroscopy; LT, leukotriene; LTC4S, leukotriene C4 synthase; MC, mast cell; MEK, mitogen-activated protein kinase kinase; MIP-1β, macrophage inflammatory protein 1β; MOX, methoxylamine; p90RSK, 90-kDa ribosomal S6 kinase; PGD2, prostaglandin D2; PGDS, PGD2 synthase; PI3K, phosphatidylinositol 3-kinase; PLA2, phospholipase A2; PPAR, peroxisome proliferator-activated receptor; PTX, pertussis toxin; RT, reverse transcriptase; SCF, stem cell factor; shRNA, short hairpin RNA; siRNA, small interfering RNA; TNF-α, tumor necrosis factor-α.
Y. Kanaoka, unpublished data.
References
- 1.Kanaoka, Y., and Boyce, J. A. (2004) J. Immunol. 173 1503–1510 [DOI] [PubMed] [Google Scholar]
- 2.Clark, J. D., Lin, L. L., Kriz, R. W., Ramesha, C. S., Sultzman, L. A., Lin, A. Y., Milona, N., and Knopf, J. L. (1991) Cell 65 1043–1051 [DOI] [PubMed] [Google Scholar]
- 3.Dixon, R. A., Diehl, R. E., Opas, E., Rands, E., Vickers, P. J., Evans, J. F., Gillard, J. W., and Miller, D. K. (1990) Nature 343 282–284 [DOI] [PubMed] [Google Scholar]
- 4.Malaviya, R., Malaviya, R., and Jakschik, B. A. (1993) J. Biol. Chem. 268 4939–4944 [PubMed] [Google Scholar]
- 5.Lam, B. K., Penrose, J. F., Freeman, G. J., and Austen, K. F. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 7663–7667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson, D. W., Ali, A., Vaillancourt, J. P., Calaycay, J. R., Mumford, R. A., Zamboni, R. J., and Ford-Hutchinson, A. W. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 2015–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leier, I., Jedlitschky, G., Buchholz, U., Cole, S. P., Deeley, R. G., and Keppler, D. (1994) J. Biol. Chem. 269 27807–27810 [PubMed] [Google Scholar]
- 8.Carter, B. Z., Shi, Z. Z., Barrios, R., and Lieberman, M. W. (1998) J. Biol. Chem. 273 28277–28285 [DOI] [PubMed] [Google Scholar]
- 9.Lee, C. W., Lewis, R. A., Corey, E. J., and Austen, K. F. (1983) Immunology 48 27–35 [PMC free article] [PubMed] [Google Scholar]
- 10.Drazen, J. M., O'Brien, J., Sparrow, D., Weiss, S. T., Martins, M. A., Israel, E., and Fanta, C. H. (1992) Am. Rev. Respir. Dis. 146 104–108 [DOI] [PubMed] [Google Scholar]
- 11.Davidson, A. B., Lee, T. H., Scanlon, P. D., Solway, J., McFadden, E. R., Jr., Ingram, R. H., Jr., Corey, E. J., Austen, K. F., and Drazen, J. M. (1987) Am. Rev. Respir. Dis. 135 333–337 [DOI] [PubMed] [Google Scholar]
- 12.Drazen, J. M., and Austen, K. F. (1987) Am. Rev. Respir. Dis. 136 985–998 [DOI] [PubMed] [Google Scholar]
- 13.Christie, P. E., Hawksworth, R., Spur, B. W., and Lee, T. H. (1992) Am. Rev. Respir. Dis. 146 1506–1510 [DOI] [PubMed] [Google Scholar]
- 14.Wenzel, S. E., Larsen, G. L., Johnston, K., Voelkel, N. F., and Westcott, J. Y. (1990) Am. Rev. Respir. Dis. 142 112–119 [DOI] [PubMed] [Google Scholar]
- 15.Altman, L. C., Munk, Z., Seltzer, J., Noonan, N., Shingo, S., Zhang, J., and Reiss, T. F. (1998) J. Allergy Clin. Immunol. 102 50–56 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton, A., Faiferman, I., Stober, P., Watson, R. M., and O'Byrne, P. M. (1998) J. Allergy Clin. Immunol. 102 177–183 [DOI] [PubMed] [Google Scholar]
- 17.Israel, E., Cohn, J., Dube, L., and Drazen, J. M. (1996) J. Am. Med. Assoc. 275 931–936 [PubMed] [Google Scholar]
- 18.Beller, T. C., Friend, D. S., Maekawa, A., Lam, B. K., Austen, K. F., and Kanaoka, Y. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beller, T. C., Maekawa, A., Friend, D. S., Austen, K. F., and Kanaoka, Y. (2004) J. Biol. Chem. 279 46129–46134 [DOI] [PubMed] [Google Scholar]
- 20.Kim, D. C., Hsu, F. I., Barrett, N. A., Friend, D. S., Grenningloh, R., Ho, I. C., Al-Garawi, A., Lora, J. M., Lam, B. K., Austen, K. F., and Kanaoka, Y. (2006) J. Immunol. 176 4440–4448 [DOI] [PubMed] [Google Scholar]
- 21.Heise, C. E., O'Dowd, B. F., Figueroa, D. J., Sawyer, N., Nguyen, T., Im, D. S., Stocco, R., Bellefeuille, J. N., Abramovitz, M., Cheng, R., Williams, D. L., Jr., Zeng, Z., Liu, Q., Ma, L., Clements, M. K., Coulombe, N., Liu, Y., Austin, C. P., George, S. R., O'Neill, G. P., Metters, K. M., Lynch, K. R., and Evans, J. F. (2000) J. Biol. Chem. 275 30531–30536 [DOI] [PubMed] [Google Scholar]
- 22.Lynch, K. R., O'Neill, G. P., Liu, Q., Im, D. S., Sawyer, N., Metters, K. M., Coulombe, N., Abramovitz, M., Figueroa, D. J., Zeng, Z., Connolly, B. M., Bai, C., Austin, C. P., Chateauneuf, A., Stocco, R., Greig, G. M., Kargman, S., Hooks, S. B., Hosfield, E., Williams, D. L., Jr., Ford-Hutchinson, A. W., Caskey, C. T., and Evans, J. F. (1999) Nature 399 789–793 [DOI] [PubMed] [Google Scholar]
- 23.Figueroa, D. J., Borish, L., Baramki, D., Philip, G., Austin, C. P., and Evans, J. F. (2003) Clin. Exp. Allergy 33 1380–1388 [DOI] [PubMed] [Google Scholar]
- 24.Ciana, P., Fumagalli, M., Trincavelli, M. L., Verderio, C., Rosa, P., Lecca, D., Ferrario, S., Parravicini, C., Capra, V., Gelosa, P., Guerrini, U., Belcredito, S., Cimino, M., Sironi, L., Tremoli, E., Rovati, G. E., Martini, C., and Abbracchio, M. P. (2006) EMBO J. 25 4615–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauvreau, G. M., Parameswaran, K. N., Watson, R. M., and O'Byrne, P. M. (2001) Am. J. Respir. Crit. Care Med. 164(8 Pt 1), 1495–1500 [DOI] [PubMed] [Google Scholar]
- 26.Laitinen, L. A., Laitinen, A., Haahtela, T., Vilkka, V., Spur, B. W., and Lee, T. H. (1993) Lancet 341 989–990 [DOI] [PubMed] [Google Scholar]
- 27.Arm, J. P., O'Hickey, S. P., Hawksworth, R. J., Fong, C. Y., Crea, A. E., Spur, B. W., and Lee, T. H. (1990) Am. Rev. Respir. Dis. 142 1112–1118 [DOI] [PubMed] [Google Scholar]
- 28.Christie, P. E., Schmitz-Schumann, M., Spur, B. W., and Lee, T. H. (1993) Eur. Respir. J. 6 1468–1473 [PubMed] [Google Scholar]
- 29.O'Hickey, S. P., Hawksworth, R. J., Fong, C. Y., Arm, J. P., Spur, B. W., and Lee, T. H. (1991) Am. Rev. Respir. Dis. 144 1053–1057 [DOI] [PubMed] [Google Scholar]
- 30.Lee, T. H., Austen, K. F., Corey, E. J., and Drazen, J. M. (1984) Proc. Natl. Acad. Sci. U. S. A. 81 4922–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurish, M. F., and Boyce, J. A. (2006) J. Allergy Clin. Immunol. 117 1285–1291 [DOI] [PubMed] [Google Scholar]
- 32.Wedemeyer, J., Tsai, M., and Galli, S. J. (2000) Curr. Opin. Immunol. 12 624–631 [DOI] [PubMed] [Google Scholar]
- 33.Mellor, E. A., Frank, N., Soler, D., Hodge, M. R., Lora, J. M., Austen, K. F., and Boyce, J. A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 11589–11593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellor, E. A., Maekawa, A., Austen, K. F., and Boyce, J. A. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 7964–7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang, Y., Borrelli, L. A., Kanaoka, Y., Bacskai, B. J., and Boyce, J. A. (2007) Blood 110 3263–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellor, E. A., Austen, K. F., and Boyce, J. A. (2002) J. Exp. Med. 195 583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang, Y., Kanaoka, Y., Feng, C., Nocka, K., Rao, S., and Boyce, J. A. (2006) J. Immunol. 177 2755–2759 [DOI] [PubMed] [Google Scholar]
- 38.Kirshenbaum, A. S., Akin, C., Wu, Y., Rottem, M., Goff, J. P., Beaven, M. A., Rao, V. K., and Metcalfe, D. D. (2003) Leuk. Res. 27 677–682 [DOI] [PubMed] [Google Scholar]
- 39.Ochi, H., Hirani, W. M., Yuan, Q., Friend, D., Austen, K. F., and Boyce, J. A. (1999) J. Exp. Med. 190 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarini, S., Gijon, M. A., Folco, G., and Murphy, R. C. (2006) J. Biol. Chem. 281 10134–10146 [DOI] [PubMed] [Google Scholar]
- 41.Ziouzenkova, O., Perrey, S., Asatryan, L., Hwang, J., MacNaul, K. L., Moller, D. E., Rader, D. J., Sevanian, A., Zechner, R., Hoefler, G., and Plutzky, J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2730–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivares-Reyes, J. A., Shah, B. H., Hernandez-Aranda, J., Garcia-Caballero, A., Farshori, M. P., Garcia-Sainz, J. A., and Catt, K. J. (2005) Mol. Pharmacol. 68 356–36443 [DOI] [PubMed] [Google Scholar]
- 43.Brown, J. D., and Plutzky, J. (2007) Circulation 115 518–533 [DOI] [PubMed] [Google Scholar]
- 44.Kliewer, S. A., Lenhard, J. M., Willson, T. M., Patel, I., Morris, D. C., and Lehmann, J. M. (1995) Cell 83 813–819 [DOI] [PubMed] [Google Scholar]
- 45.Pawliczak, R., Logun, C., Madara, P., Lawrence, M., Woszczek, G., Ptasinska, A., Kowalski, M. L., Wu, T., and Shelhamer, J. H. (2004) J. Biol. Chem. 279 48550–48561 [DOI] [PubMed] [Google Scholar]
- 46.Kim, E. J., Park, K. S., Chung, S. Y., Sheen, Y. Y., Moon, D. C., Song, Y. S., Kim, K. S., Song, S., Yun, Y. P., Lee, M. K., Oh, K. W., Yoon, D. Y., and Hong, J. T. (2003) J Pharmacol. Exp. Ther. 307 505–517 [DOI] [PubMed] [Google Scholar]
- 47.Rokos, C. L., and Ledwith, B. J. (1997) J. Biol. Chem. 272 13452–13457 [DOI] [PubMed] [Google Scholar]
- 48.Wada, K., Nakajima, A., Katayama, K., Kudo, C., Shibuya, A., Kubota, N., Terauchi, Y., Tachibana, M., Miyoshi, H., Kamisaki, Y., Mayumi, T., Kadowaki, T., and Blumberg, R. S. (2006) J. Biol. Chem. 281 12673–12681 [DOI] [PubMed] [Google Scholar]
- 49.Gardner, O. S., Dewar, B. J., Earp, H. S., Samet, J. M., and Graves, L. M. (2003) J. Biol. Chem. 278 46261–46269 [DOI] [PubMed] [Google Scholar]
- 50.Bagga, S., Price, K. S., Lin, D., Friend, D. S., Austen, K. F., and Boyce, J. A. (2004) Blood 104 4080–4087 [DOI] [PubMed] [Google Scholar]
- 51.Fuller, R. W., Dixon, C. M., Dollery, C. T., and Barnes, P. J. (1986) Am. Rev. Respir. Dis. 133 252–254 [DOI] [PubMed] [Google Scholar]
- 52.Mamedova, L., Capra, V., Accomazzo, M. R., Gao, Z. G., Ferrario, S., Fumagalli, M., Abbracchio, M. P., Rovati, G. E., and Jacobson, K. A. (2005) Biochem. Pharmacol. 71 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kliewer, S. A., Forman, B. M., Blumberg, B., Ong, E. S., Borgmeyer, U., Mangelsdorf, D. J., Umesono, K., and Evans, R. M. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 7355–735954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodera, Y., Takeyama, K., Murayama, A., Suzawa, M., Masuhiro, Y., and Kato, S. (2000) J. Biol. Chem. 275 33201–33204 [DOI] [PubMed] [Google Scholar]
- 55.Pontsler, A. V., St. Hilaire, A. V., Marathe, G. K., Zimmerman, G. A., and McIntyre, T. M. (2002) J. Biol. Chem. 277 13029–13036 [DOI] [PubMed] [Google Scholar]
- 56.Hardy, C. C., Robinson, C., Tattersfield, A. E., and Holgate, S. T. (1984) N. Engl. J. Med. 311 209–213 [DOI] [PubMed] [Google Scholar]
- 57.Hirai, H., Tanaka, K., Yoshie, O., Ogawa, K., Kenmotsu, K., Takamori, Y., Ichimasa, M., Sugamura, K., Nakamura, M., Takano, S., and Nagata, K. (2001) J. Exp. Med. 193 255–26160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bochenek, G., Nagraba, K., Nizankowska, E., and Szczeklik, A. (2003) J. Allergy Clin. Immunol. 111 743–749 [DOI] [PubMed] [Google Scholar]
- 59.Adamjee, J., Suh, Y. J., Park, H. S., Choi, J. H., Penrose, J. F., Lam, B. K., Austen, K. F., Cazaly, A. M., Wilson, S. J., and Sampson, A. P. (2006) J Pathol. 209 392–399 [DOI] [PubMed] [Google Scholar]
- 60.Sousa, A. R., Parikh, A., Scadding, G., Corrigan, C. J., and Lee, T. H. (2002) N. Engl. J. Med. 347 1493–1499 [DOI] [PubMed] [Google Scholar]
- 61.Cowburn, A. S., Sladek, K., Soja, J., Adamek, L., Nizankowska, E., Szczeklik, A., Lam, B. K., Penrose, J. F., Austen, F. K., Holgate, S. T., and Sampson, A. P. (1998) J. Clin. Investig. 101 834–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sousa, A., Pfister, R., Christie, P. E., Lane, S. J., Nasser, S. M., Schmitz-Schumann, M., and Lee, T. H. (1997) Thorax 52 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]