Abstract
Cyclic AMP (cAMP)-dependent processes are pivotal during the early stages of adipocyte differentiation. We show that exchange protein directly activated by cAMP (Epac), which functions as a guanine nucleotide exchange factor for the Ras-like GTPases Rap1 and Rap2, was required for cAMP-dependent stimulation of adipocyte differentiation. Epac, working via Rap, acted synergistically with cAMP-dependent protein kinase (protein kinase A [PKA]) to promote adipogenesis. The major role of PKA was to down-regulate Rho and Rho-kinase activity, rather than to enhance CREB phosphorylation. Suppression of Rho-kinase impaired proadipogenic insulin/insulin-like growth factor 1 signaling, which was restored by activation of Epac. This interplay between PKA and Epac-mediated processes not only provides novel insight into the initiation and tuning of adipocyte differentiation, but also demonstrates a new mechanism of cAMP signaling whereby cAMP uses both PKA and Epac to achieve an appropriate cellular response.
Adipocytes are derived from multipotent mesenchymal stem cells in a process involving commitment to the adipocyte lineage followed by terminal differentiation of the committed preadipocytes. The process is regulated via complex interaction of external and internal clues, where cell shape and cytoskeletal tension converging on regulation of Rho and Rho-kinase activity have been demonstrated to play pivotal roles (48, 63). Whereas our understanding of the early steps of lineage determination still is limited, regulatory cascades controlling terminal adipocyte differentiation have been elucidated in great detail, particularly the sequential action of different transcription factors culminating in the expression of adipocyte-specific genes (25, 30, 58). Much information on terminal adipocyte differentiation has been obtained using model cell lines such as 3T3-L1 and 3T3-F442A or mouse embryo fibroblasts (MEFs). In both MEFs and 3T3-L1 preadipocytes, terminal differentiation is initiated upon treatment with fetal calf serum, glucocorticoids, and high levels of insulin or physiological concentrations of insulin-like growth factor 1 (IGF-1). Factors that increase cellular cyclic AMP (cAMP), such as isobutylmethylxanthine (IBMX) or forskolin, strongly accelerate the initiation of the differentiation program (for review, see references 25 and 45).
Elevation of cellular cAMP concentration has been associated with crucial events in the early program of differentiation, such as suppression of Wnt10b (5) and Sp1 (64) and induction of CCAAT/enhancer-binding protein β (C/EBPβ) (10, 29, 70). Moreover, the transcriptional activity of peroxisome proliferator-activated receptor δ (PPARδ) is regulated synergistically by ligands and cAMP (32). In addition, cAMP has been implicated in the production of endogenous PPARγ ligand(s) occurring during the initial stages of differentiation (46, 67). The cAMP-responsive element-binding protein (CREB) is a central transcriptional activator of the adipocyte differentiation program. Activated CREB induces expression of C/EBPβ, triggering expression of a number of transcription factors, including C/EBPα and PPARγ (16, 64-66, 70, 72). Indeed, forced expression of constitutively active CREB can induce adipogenesis, whereas expression of a dominant-negative form of CREB blocks differentiation (56). The importance of CREB is underscored by the finding that adipocyte differentiation of CREB-deficient mouse embryo fibroblast is impaired (72) and that small interfering RNA-mediated depletion of CREB and the closely related activating transcription factor 1 (ATF1) blocks adipocyte differentiation (26). CREB was initially characterized as a cAMP target whose transcriptional activity was stimulated by cAMP-dependent protein kinase (protein kinase A [PKA])-catalyzed phosphorylation on serine 133 (28), but insulin (Ins) signaling may also activate CREB in 3T3-L1 cells through Ser-133 phosphorylation via the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway (40).
While cAMP signaling via PKA has been investigated for decades, the complexity of cAMP signaling via interplay between PKA and the exchange proteins directly activated by cAMP (Epac1 and Epac2) is only beginning to be understood. Epac1 and Epac2 function as guanine nucleotide exchange factors (GEFs) for the Ras-like small GTPases Rap1 and Rap2 (6), and possibly Rit (60), and several cAMP-dependent processes are now believed to be modulated by Epac. Epac may mediate cAMP-dependent exocytosis (36, 37, 52) and integrin-dependent cell adhesion (17, 24, 54). Whereas Epac and PKA can exert opposing effects in regulating downstream targets such as protein kinase B (PKB) (49), they act synergistically to promote PC-12 cell differentiation, as judged by neurite extension (15).
The present work was undertaken to determine if Epac had any role in cAMP-stimulated adipocyte differentiation of 3T3-L1 preadipocytes and, if so, to dissect the contributions of Epac and PKA. We demonstrate that cAMP stimulated adipocyte differentiation through the concerted action of PKA and Epac/Rap. A similar finding was made for cAMP-stimulated adipocyte differentiation of MEFs. While stimulation of PKA activity was not required for the increased phosphorylation of CREB during the initiation of adipocyte differentiation, it was important for the suppression of Rho/Rho-kinase activity. Inhibition of Rho-kinase activity in 3T3-L1 preadipocytes decreased Ins/IGF-1 signaling, but concomitant activation of Epac restored Ins/IGF-1 sensitivity. Accordingly, adipocyte differentiation was still Epac dependent when Rho-kinase was inhibited, whereas PKA activity was dispensable under such conditions. This interplay between PKA-, Epac-, and Rho-kinase-mediated processes provides novel insight into regulatory circuits controlling the initiation of adipocyte differentiation and provides a new example of how cAMP can use both PKA and Epac to achieve an appropriate cellular response.
MATERIALS AND METHODS
Plasmids.
The retroviral expression plasmid encoding dominant-negative Rap1A (pBABE-Rap1-N17) was constructed by inserting the BamHI/XhoI fragment of pcDNA3-HA-Rap1A-N17 (kindly provided by Eva Pålsson-McDermott) into the BamHI/SalI sites of a polylinker-modified plasmid, pBABE-puro (kindly provided by Ormond MacDougald). The plasmid encoding dominant-negative RhoA for retroviral expression (pLXSN-RhoA-N19) was constructed by inserting the HindIII/NotI fragment from pcDNA3-RhoA-N19 (kindly provided by R. Regazzi) into the HindIII/NotI sites of pLXSN (kindly provided by Ormond MacDougald). The vector encoding dominant-negative Epac1 (dnEpac1; Gly269-to-Glu mutation) for retroviral expression (pLXSN-dnEpac1) was constructed by insertion of the EcoRI (blunt ended)/NotI fragment of pGEX-dnEpac1 (kindly provided by Johannes Bos) into the HpaI/NotI sites of pLXSN. pMT2-HA-RapGAP (55) was kindly provided by Johannes L. Bos. pBABE-RapGAP was generated by subcloning full-length Rap-GTPase-activating (RapGAP; BglII/XbaI blunt) fragment from pMT2-HA-RapGAP into BamHI/HpaI-digested pBABE-Puro.IpJim-RIαDN, and pJim was a kind gift from Reidun Kopperud.
Cell culture and differentiation.
3T3-L1 cells were cultured to confluence in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum. Two-days-postconfluent (designated day 0) cells were induced to differentiate with DMEM supplemented with 10% fetal bovine serum (FBS) and 1 μM dexamethasone (Dex) (Sigma). One microgram per milliliter Ins (Sigma) or IGF-1 (Sigma), 0.5 mM IBMX (Sigma), 100 μM 8-para-chloro-phenylthio-cAMP (8-CPT-cAMP), 200 μM 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-Me-cAMP) (Biolog), 100 μM N6-monobutyryl-cAMP (6-MB-cAMP) (Biolog), and/or 100 μM N6-benzoyl-cAMP (6-Bnz-cAMP) was included as indicated in the figure legends. After 48 h, the media were replaced with DMEM supplemented with 10% FBS and 1 μg/ml Ins or IGF-1 as indicated in the figure legends. The cells were subsequently refed every 48 h with DMEM supplemented with 10% fetal bovine serum. When included, H-89 (10 μM) (Biomol), sc-3536 (10 μM), (Santa Cruz), and Rp-8-Br-cAMPS/Rp-cAMPS (100 μM) (Biolog) were present from day 0 to day 2. The preparation of MEFs has been described previously (44). MEFs were grown in AmnioMax basal medium (Life Technologies, Inc.) supplemented with 7.5% FBS, 7.5% AmnioMax-C100 supplement, and 2 mM glutamine and were induced to differentiate as 3T3-L1 cells. Staining of lipid by oil red O was performed as described previously (32).
Retroviral transduction.
Phoenix-Eco cells were plated at 30 to 40% confluence in DMEM supplemented with 10% FBS. Next day, the cells were transfected using a standard calcium phosphate method by adding 10 μg retroviral expression vector (pJim-RIαDN, pLXSN-dnEpac1, pLXSN-RhoA-N19, pBABE-Rap1N17, or the empty retroviral vectors) and 15 μg pBSK (Stratagene) to a total of 25 μg DNA per 9-cm dish. Two days posttransfection, the virus-containing media were collected by centrifugation and immediately used to infect 30 to 40% confluent 3T3-L1 cells by mixing viral supernatant 1:1 with DMEM supplemented with 10% calf serum. Polybrene (Sigma) was added to a final concentration. of 7 μg/ml. After 24 h, the transduced cells were split and subjected to selection (400 μg/ml hygromycin B [Calbiochem] or 3 μg/ml puromycin). After approximately 4 days, the selected clones were pooled and replated for differentiation.
Real-time RT-PCR.
Total RNA was purified from cells using Trizol, and cDNA was synthesized as described earlier (46) and quantified by real-time quantitative PCR (qPCR) using the ABI PRISM 7700 sequence detection system (Applied Biosystems). Each PCR mixture contained, in a final volume of 25 μl, 1 μl of first-strand cDNA, 12.5 μl of 2× Sybr green PCR master mix, and 5 pmol of each primer. All reactions were performed using the following cycling conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. PCR was carried out in 96-well plates and in duplicate. Primers for real-time reverse transcription-PCR (RT-PCR) were designed using Primer Express 2.0 (Applied Biosystems). Target gene mRNA expression was normalized to transcription factor IIB (TFIIB) or TATA-binding protein (TBP) mRNA expression, and the relative amounts of all mRNAs were calculated. The following primers were used (upstream and downstream, respectively): Epac1, 5′-GGGACTCCGCTGGACACC and 5′-CGGCCAGAGCAGCAATGCCG; Epac2, 5′-CAATCGGATTCTGAGGGACG and 5′-CATTTAAAACCGAATCTG; C/EBPα, 5′-CAAGAACAGCAACGAGTACCG and 5′-GTCACTGGTCAACTCCAGCAC; aP2, 5′-CTGGGCGTGGAATTCGAT and 5′-GCTCTTCACCTTCCTGTCGTCT; PPARγ2, 5′-ACAGCAAATCTCTGTTTTATGC and 5′-TGCTGGAGAAATCAACTGTGG; LXRα, 5′-GAGTTGTGGAAGACAGAACCTCAA and 5′-GGGCATCCTGGCTTCCTC; and TBP, 5′-ACCCTTCACCAATGACTCCTATG and 5′-ATGATGACGGCAGCAAATCGC.
Western blotting.
Obtaining whole-cell extracts, electrophoresis, blotting, visualization, and stripping of membranes were performed as described previously (31). The primary antibodies used were obtained from the following sources: Cell Signaling Technology, mouse anti phospho-ERK1/2 (Thr-202/Tyr-204), rabbit anti-ERK1/2, rabbit anti-phospho-PKB (Ser-473), rabbit anti-PKB, mouse anti-CREB, mouse anti-phospho-myosin light chain (MLC [Ser-19]), and rabbit anti-MLC; Upstate, mouse anti-phospho-CREB (Ser-133); Cayman Chemical, rabbit anti FABP4/aP2; and Santa Cruz, rabbit anti-PPARγ and rabbit anti-TFIIB. The secondary antibodies were horseradish peroxidase-conjugated antimouse or antirabbit antibodies obtained from DAKO.
Determination of PKA activity in cell lysates.
Cells were incubated for 15 min with various agents supposed to modulate their cAMP level or PKA activity, washed in ice-cold phosphate-buffered saline, and lysed in 0.5 ml of 50 mM potassium phosphate buffer (pH 7.0) with 1 mM EGTA, 0.3 mM EDTA, 2 mM 1,4-dithioerythriol (DTE), 0.15% Triton X-100, and Complete protease inhibitor cocktail (Roche). Lysates were snap-frozen in liquid nitrogen and thawed immediately before assay of kinase activity, which was performed essentially as described by Ekanger et al. (22). Briefly, incubations were for 5 to 10 min at 30°C in 15 mM HEPES-NaOH, 50 mM potassium phosphate, 10 mM magnesium acetate, 0.5 mM EGTA, 100 μM [γ-32P]ATP, and 70 μM kemptide (LRRASLG) substrate. Some incubations contained cAMP, cAMP analogs, or kinase inhibitors (see the relevant figure legend for details). The blank value was determined by incubating in the presence of 100 nM of the inhibitor peptide from the heat-stable PKA inhibitor.
Other assays.
Rap1 and Rho activity were measured using the Rap1 activation assay kit (no. 17-321; Upstate) and the Rho activity kit (no. 17-294; Upstate).
Statistical analysis.
Statistical evaluation of the data was performed using Student t test. A P value of <0.05 was considered to be significant. n denotes the number of independent assays or experiments.
RESULTS
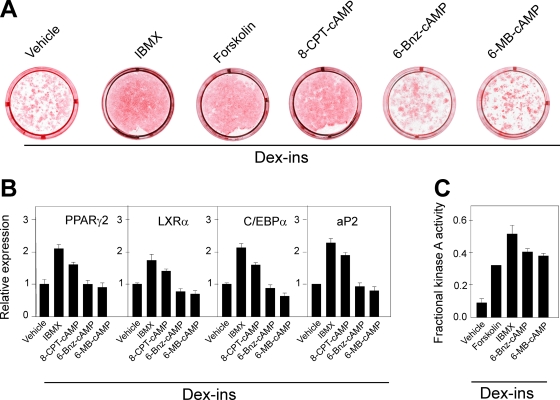
In accordance with previous studies (for review, see references 25 and 45), we observed that an increase in the cellular level of cAMP by the addition of IBMX or forskolin accelerated adipocyte differentiation of 3T3-L1 cells treated with Dex and Ins. A similar stimulation of adipogenesis was observed in response to the cell-permeable cAMP analog 8-CPT-cAMP, which activates both Epac and PKA (14). In contrast, cAMP analogs 6-MB-cAMP and 6-Bnz-cAMP, which selectively activate PKA (14, 15), were inefficient, whether judged by oil red O staining or adipocyte marker gene expression (Fig. 1A and B). To demonstrate that the presumed PKA activators efficiently activated PKA in 3T3-L1 cells, we directly determined the PKA activity in lysates obtained from cells treated for 15 min with vehicle IBMX, forskolin, 6-MB-cAMP, or 6-Bnz-cAMP. Both 6-MB-cAMP and 6-Bnz-cAMP led to significant activation of PKA to a degree comparable to that observed when the cells were treated with forskolin or IBMX. These findings suggested that activation of PKA alone was insufficient to promote adipogenesis, challenging the prevailing notion that cAMP stimulates adipogenesis entirely via activation of PKA leading to enhanced phosphorylation and activation of CREB (56, 57, 72).
FIG. 1.
Elevation of cAMP, but not selective activation of PKA, accelerates differentiation of 3T3-L1 preadipocytes. (A) Two-day-postconfluent 3T3-L1 cells were induced to differentiate by treatment with 1 μM Dex and 1 μg/ml Ins in the presence of 0.5 mM IBMX, 10 μM forskolin, 100 μM 8-CPT-cAMP, 100 μM 6-MB-cAMP, or 100 μM 6-Bnz-cAMP, as described in Materials and Methods. The cells were stained with oil red O and photographed on day 8. (B) RNA was isolated on day 8, and expression of PPARγ2, LXRα, C/EBPα, and aP2 was determined by RT-qPCR. The error bars represent standard deviations (n = 3). (C) Relative PKA activity in lysates of 3T3-L1 cells pretreated for 15 min in medium containing Dex plus Ins with vehicle, 0.5 mM IBMX, 50 μM forskolin, or 100 μM 6MB-cAMP. The error bars represent standard errors of the means (n = 3 to 5).
We studied therefore whether cAMP-mediated activation of Epac1 or Epac2 might be required for the adipogenic effect of cAMP. To determine if Epac1 and/or Epac 2 was expressed in the 3T3-L1 cells, specific primer sets were used for real-time qPCR. While Epac2 mRNA was undetectable, Epac1 mRNA was expressed in 2-day-postconfluent preadipocytes (designated day 0 in the differentiation process) at a level about threefold higher than that in mouse liver (Fig. 2A). Upon induction of differentiation, the level of Epac1 mRNA declined rapidly during the first 24 h to stabilize at a level approximately 45% of that in day 0 cells. In contrast, Epac2 mRNA was below the detection limit in undifferentiated as well as differentiated 3T3-L1 cells. The Epac2 primer set was validated using liver mRNA as a positive control (Fig. 2A and B). Analysis of mRNA isolated from the stromal vascular fraction (SVF) of epididymal white adipose tissue (eWAT) and interscapular brown adipose tissue (iBAT) similarly revealed that Epac1 was more highly expressed in the SVF than in the adipocyte fraction, whereas Epac2 was undetectable (Fig. 2B).
FIG. 2.
Expression of Epac in 3T3-L1 cells and mouse adipose tissues. (A) RNA was isolated on days 0, 1, 2, 3, 4, 6, and 10 from 3T3-L1 cells induced to differentiate by Dex, Ins, and IBMX and from mouse liver. (B) RNA was extracted from SVF or mature adipocytes (Ads) isolated from mouse eWAT, iBAT, and liver as described in Materials and Methods. The expression of Epac1 and Epac2 was determined using RT-qPCR and normalized to TBP expression. The error bars represent standard deviations (n = 3).
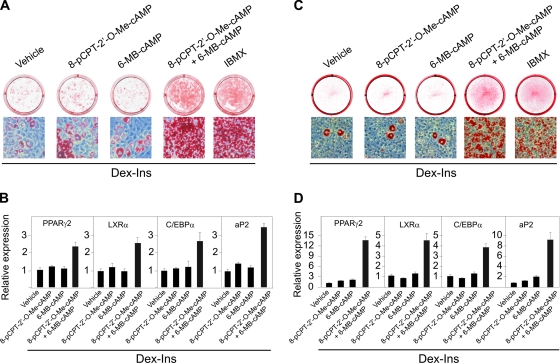
Having established that Epac1 is expressed in 3T3-L1 cells, we tested whether selective Epac-activating cAMP analogs like 8-pCPT-2′-O-Me-cAMP (15, 23) could mimic the effect of an increase in the endogenous level of cAMP in 3T3-L1 cells treated with Dex and Ins. This was not the case, as the majority of the cells remained fibroblast-like after treatment with the Epac-activating cAMP analogs. However, when the Epac activator 8-pCPT-2′-O-Me-cAMP was combined with the PKA activator 6-MB-cAMP in the presence of Dex and Ins more than 90% of the cells rounded up and differentiated into mature adipocytes. The degree of differentiation obtained by the combined action of the selective Epac and PKA activators was comparable to that induced by the phosphodiesterase inhibitor IBMX, as determined by oil red O staining (Fig. 3A). To analyze the effect on gene expression, 3T3-L1 preadipocytes were induced with Dex and Ins together with the Epac-activating analog 8-pCPT-2′-O-Me-cAMP and the PKA-activating analog 6-MB-cAMP. As shown in Fig. 3B, no significant induction of adipocyte marker genes was observed in cells treated with either analog alone, whereas strong induction occurred with the combined administration of both cAMP analogs.
FIG. 3.
Activation of Epac and PKA synergistically induces differentiation of 3T3-L1 cells and MEFs into adipocytes. Two-day-postconfluent 3T3-L1 cells (A) or MEFs (C) were induced to differentiate by treatment with Dex and Ins in the presence of combinations of 200 μM 8-pCPT-2′-O-Me-cAMP and 100 μM 6-MB-cAMP or 0.5 mM IBMX as indicated. The cells were stained with oil red O on day 8. RNA was isolated on day 8, and expression of PPARγ2, LXRα, C/EBPα, and aP2 in 3T3-L1 cells (B) and MEFs (D) was determined by RT-qPCR. The error bars represent standard deviations (n = 3).
In order to determine if the synergy between PKA and Epac activators was peculiar for the 3T3-L1 cell line, MEFs were tested. As evidenced by oil red O staining of lipids, activation of either Epac or PKA alone was insufficient to achieve adipose conversion. As for the 3T3-L1 cells, only combined activation of Epac and PKA stimulated adipose conversion (Fig. 3C). Similarly, strong induction of adipocyte marker gene expression required the simultaneous activation of Epac and PKA (Fig. 3D). Thus, in both models activators of Epac and PKA synergistically promoted adipogenesis.
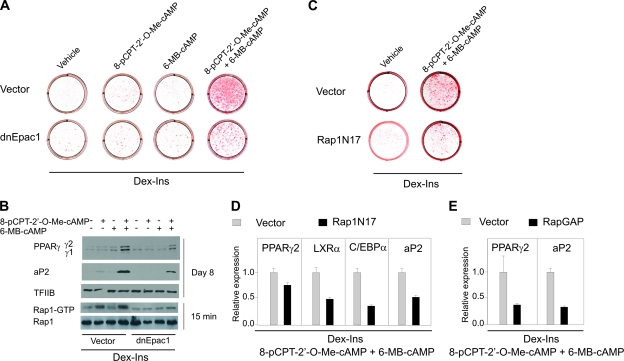
To support the results obtained by pharmacological activation of Epac1 and PKA, 3T3-L1 cells were transduced with a retroviral vector expressing a dominant-negative form of Epac1 or the empty vector and tested for their ability to undergo cAMP-stimulated adipose conversion. Control cells transduced with the empty vector differentiated when both Epac and PKA were activated by 8-pCPT-2′-O-Me-cAMP and 6-MB-cAMP in combination. In contrast, in cells expressing the dominant-negative form of Epac1, the majority of the cells remained fibroblast-like and were not stained by oil red O (Fig. 4A). Furthermore, the induction of PPARγ and adipocyte lipid-binding protein (aP2) was severely blunted in the cells transduced with dnEpac1 (Fig. 4B). Knockdown of Epac1 expression by lentivirus-mediated expression of anti-Epac1 short hairpin RNA similarly blunted differentiation of 3T3-L1 preadipocytes (data not shown).
FIG. 4.
Activation of Epac is required for differentiation of 3T3-L1 cells into adipocytes. 3T3-L1 cells were retrovirally transduced with an empty vector, a vector expressing dnEpac1 (A and B), a vector expressing a dominant-negative form of Rap1A (Rap1N17) (C and D), or a vector expressing Rap GTPase activating protein (RapGAP) (E). The cells were grown to confluence, and at 2 days postconfluence they were induced to differentiate by Dex and Ins in the presence of combinations of 200 μM 8-pCPT-2′-O-Me-cAMP and 100 μM 6-MB-cAMP as indicated in the figure. (A and C) The cells were stained with oil red O and photographed on day 8. (B) GTP-bound Rap1 was measured by a Rap1 activation pull-down assay as described in Materials and Methods. Expression of aP2 and PPARγ was determined by Western blotting on day 8. TFIIB was used for control of equal protein loading on the gel. One representative experiment out of three independent experiments is shown. (D and E) RNA was isolated on day 8, and expression of LXRα, C/EBPα (D), PPARγ2, and aP2 (D and E) was determined by RT-qPCR. The error bars represent standard deviations (n = 3).
In order to test the Epac selectivity of the cAMP analogs used and the ability of dnEpac1 to block endogenous Epac action, we determined the level of active (GTP-associated) Rap1 in the 3T3-L1 cells in response to treatment with various cAMP analogs. We found no activation of Rap1 in response to the PKA activator 6-MB-cAMP, while 8-pCPT-2′-O-Me-cAMP activated Rap1 both in the absence and presence of PKA activator. It should be noted that no activation of PKA was observed in cells treated with 8-pCPT-2′-O-Me-cAMP (see Fig. 6A). Importantly, activation of Rap1 by 8-pCPT-2′-O-Me-cAMP was abolished in cells with forced expression of dnEpac1 (Fig. 4B).
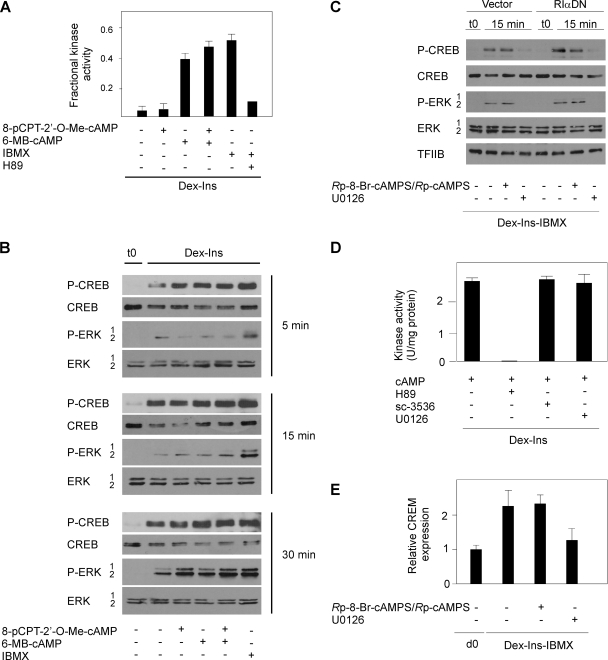
FIG. 6.
Induction of CREB phosphorylation during initiation of adipocyte differentiation is dependent on ERK1/2 activity. (A) 3T3-L1 cells were treated for 15 min in Dex-Ins medium with various combinations of 200 μM 8-pCPT-2′-O-Me-cAMP, 200 μM 6MB-cAMP, 0.5 mM IBMX, and 10 μM H89 as indicated. Subsequently the PKA activity in lysates of the cells was determined. The error bars represent standard errors of the means (n = 3). (B) 3T3-L1 preadipocytes at 2 days postconfluence were treated with Dex and Ins with combinations of 200 μM 8-pCPT-2′-O-Me-cAMP, 200 μM 6MB-cAMP, and 0.5 mM IBMX. Whole-cell extracts were prepared after 5, 15, and 30 min and analyzed for phosphorylation of CREB and ERK1/2 by Western blotting. One representative experiment out of three independent experiments is shown. (C) 3T3-L1 preadipocytes at 2 days postconfluence were treated with Dex, Ins, and IBMX with or without 100 μM Rp-8-Br-cAMPS/Rp-cAMPS or 10 μM U0126 as indicated. Whole-cell extracts were prepared after 15 min and analyzed for phosphorylation of CREB and ERK1/2 by Western blotting. One representative experiment out of three independent experiments is shown. (D) Effects of protein kinase inhibitors on PKA activity in 3T3-L1 lysates. The lysates were incubated with 10 μM H89, 10 μM sc-3536, or 10 μM U0126 in the presence of a maximally PKA-stimulating concentration of cAMP (1 μM). The error bars represent standard errors of the means (n = 3). (E) Two-day-postconfluent 3T3-L1 cells were induced to differentiate with Dex, Ins, and IBMX with or without 100 μM Rp-8-Br-cAMPS/Rp-cAMPS or 10 μM U0126. RNA was isolated after 6 h, and expression of CREB was determined by RT-qPCR. d0, day 0. The error bars represent standard deviations (n = 3).
To determine if the Epac-mediated activation of Rap was a bystander effect or was important for adipogenesis, we tested whether forced expression of the dominant-negative Rap1N17 would inhibit adipogenesis. We noted a robust adipocyte differentiation in 3T3-L1 cells transduced with vector alone, whereas adipocyte differentiation of cells expressing Rap1N17 was suppressed, whether determined by oil red O staining (Fig. 4C) or adipocyte marker gene expression (Fig. 4D). Furthermore, retroviral expression of RapGAP, which facilitates conversion of the active Rap-GTP into its inactive GDP-bound form, significantly reduced expression of the adipogenic marker genes PPARγ2 and aP2 (Fig. 4E). Collectively, these findings indicate that cAMP-dependent activation of the Epac1/Rap1 pathway is required for adipocyte differentiation.
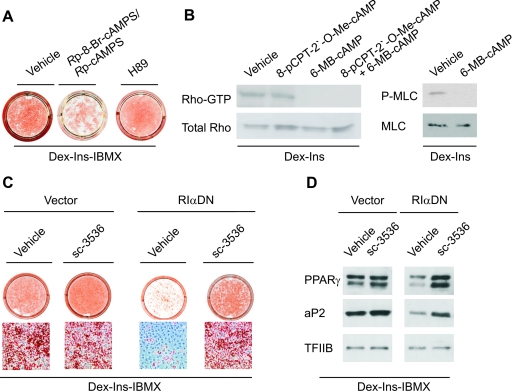
The data in the preceding paragraphs suggested that active PKA was necessary, but not sufficient, for cAMP-stimulation of adipogenesis. The role of PKA was ascertained by the demonstration that the PKA-specific inhibitory cAMP analogs of the equatorial diastereoisomer of adenosine-3′,5′-cyclic monophosphorothioate (Rp-8-Br-cAMPS/Rp-cAMPS) (15) counteracted 3T3-L1 cell differentiation (Fig. 5 A) and that differentiation was blocked by forced expression of dominant-negative RIα (Fig. 5C), which, like the Rp-cAMPS analogs, significantly decreased the PKA activity in 3T3-L1 cell extracts (data not shown). In contrast, the widely used, but nonspecific, PKA inhibitor H89 failed to abrogate cAMP-stimulated adipogenesis (Fig. 5A). This suggested that PKA could act by inhibiting an additional kinase targeted by H89. In this way H89 would make PKA superfluous for differentiation. H89 is a potent inhibitor of Rho-kinases (42), which therefore would be a prime candidate for the putative kinase inactivated downstream of PKA; the more so as inhibition of Rho-kinase constitutes a crucial step for initiation of adipocyte differentiation (51). Since the Rho-kinase is stimulated by GTP-Rho, an obvious mechanism of PKA-induced inhibition of Rho-kinase would be to convert Rho-GTP to the inactive Rho-GDP form. In fact, activation of PKA alone or in combination with activation of Epac reduced the level of Rho-GTP to undetectable levels, whereas activation of Epac alone had no effect, and furthermore, activation of PKA by 6-MB-cAMP decreased phosphorylation of the Rho-kinase substrate MLC (Fig. 5B). To further substantiate the notion that PKA activation stimulated adipogenesis via downregulation of a Rho/Rho-kinase-dependent pathway, we examined whether pharmacological inhibition of Rho-kinase was sufficient to restore adipogenesis of 3T3-L1 cells expressing the dominant-negative form of the RIα subunit. As predicted, addition of the Rho-kinase inhibitor sc-3536 restored the differentiation of cells expressing the dominant-negative RIα subunit as determined by oil red O staining (Fig. 5C) and expression of the adipocyte marker proteins PPARγ and aP2 (Fig. 5D). Based on these findings, we conclude that activation of PKA is dispensable for efficient adipocyte differentiation in the presence of Dex, high levels of Ins, and IBMX, provided that the Rho-kinase is inhibited.
FIG. 5.
Activation of PKA is required for differentiation of 3T3-L1 cells, but dispensable when Rho-kinase is inhibited. (A) 3T3-L1 cells were grown to 2 days postconfluence and induced to differentiate by a standard differentiation cocktail consisting of Dex, Ins, and IBMX. Additionally, 100 μM Rp-8-Br-cAMPS/Rp-cAMPS or 10 μM H89 was present from day 0 to day 2. On day 8, the cells were stained with oil red O and photographed. (B) GTP-bound Rho was measured by a Rho activation pull-down assay after 15 min of treatment with 200 μM 8-pCPT-2′-O-Me-cAMP and 100 μM 6-MB-cAMP alone or in combination. Phosphorylated MLC and MLC were determined by Western blotting after 15 min of treatment with 100 μM 6-MB-cAMP or vehicle. One representative experiment out of three independent experiments is shown. (C) 3T3-L1 cells were retrovirally transduced with an empty vector or a vector expressing a dominant-negative form of the RIα PKA subunit (RIαDN). At 2 days postconfluence, the cells were induced to differentiate by Dex, IBMX, and Ins in the absence and presence of 10 μM sc-3536. On day 8, the cells were stained with oil red O and photographed or whole-cell extracts were prepared and the expression of PPARγ and aP2 was determined by Western blotting using TFIIB as a control for equal protein loading. One representative experiment out of three independent experiments is shown (D).
The finding that the stimulatory effect of PKA activation on adipocyte differentiation could be mimicked by Rho-kinase inhibition and the fact that the dual PKA and Rho-kinase inhibitor enhanced rather than prevented adipocyte differentiation questioned the importance of PKA-mediated phosphorylation of CREB in cAMP-stimulated adipocyte differentiation. To investigate this, we first determined the effects of 6-MB-cAMP, 8-pCPT-2′-O-Me-cAMP, forskolin, IBMX, and H89 on the PKA activity in 3T3-L1 cells. The cells were treated for 15 min, as indicated in Fig. 6A, and PKA activity in the lysates was determined. As expected, the addition of 8-pCPT-2′-O-Me-cAMP did not increase the PKA activity, whereas 6-MB-cAMP, forskolin, and IBMX robustly increased PKA activity. The combination of 6-MB-cAMP with 8-pCPT-2′-O-Me-cAMP increased PKA activity to a level comparable to that observed with 6-MB-cAMP alone. Finally, addition of H89 completely prevented the IBMX-dependent increase in PKA activity (Fig. 6A). Next, to determine to what extent activation of PKA affected phosphorylation of CREB on Ser-133, 3T3-L1 cells were grown to 2 days postconfluence and then induced with DMEM containing 10% FBS in the absence or presence of cAMP analogs. Cells were harvested after 5, 15, and 30 min. Figure 6A demonstrates the time course of CREB and mitogen-activated protein kinase (MAPK) phosphorylation. Phosphorylation of both CREB and ERK1/2 was stimulated as early as 5 min after induction in the absence of cAMP analogs or IBMX. Maximal phosphorylation of CREB was observed after 15 min, whereas ERK1/2 phosphorylation still increased by 30 min. Interestingly, ERK1/2 phosphorylation increased rapidly in the cells treated with IBMX. Furthermore, the level of phosphorylated ERK1/2 after 15 and 30 min tended to be higher in cells receiving IBMX or the Epac activator 8-pCPT-2′-O-Me-cAMP alone or in combination with 6-MB-cAMP, possibly reflecting an enhanced Ins-dependent signaling (see below). No significant difference in CREB phosphorylation was observed in cells treated with IBMX, 6-MB-cAMP, 8-pCPT-2′-O-Me-cAMP, or 6-MB-cAMP plus 8-pCPT-2′-O-Me-cAMP (Fig. 6B). These findings indicated that robust CREB phosphorylation could be induced without specific activation of PKA, but might depend on ERK1/2 activation. To know if this could be the case, 3T3-L1 cells were transduced with either an empty vector or a vector expressing the dominant-negative form of RIα and induced for 15 min with Dex, Ins, and IBMX, in the absence or presence of the selective PKA inhibitor Rp-cAMPS or the MEK inhibitor U0126. Cell extracts were analyzed for CREB and MAPK phosphorylation by Western blotting. Figure 6C demonstrates that phosphorylation of CREB and ERK1/2 was induced also when the PKA inhibitor was present and in cells expressing the dominant-negative form of RIα. In contrast, the MEK inhibitor almost completely prevented the increased phosphorylation of not only ERK1/2, but also CREB. In vitro kinase assays demonstrated that the MEK kinase inhibitor (and the Rho-kinase inhibitor) did not inhibit PKA, whereas H89 potently inhibited PKA (Fig. 6D). Finally, we demonstrated that expression of a canonical CREB-responsive gene was induced by Dex, Ins, and IBMX in the presence of the PKA inhibitor Rp-cAMPS, but not in the presence of the MEK inhibitor (Fig. 6E).
CREB has been shown to play a pivotal role during initiation of adipocyte differentiation, at least in part by regulating the expression of C/EBPβ (72), although alternative routes for induction of C/EBPβ also seem to exist (26). It is well established that phosphorylation of Ser-133 is necessary, but not sufficient, for CREB-mediated transactivation and that additional PKA-dependent processes may be needed for transcriptional activation (8). However, taken together all experiments described above argue against PKA being directly involved in CREB phosphorylation and activation. Rather, the activation of CREB and the ensuing induction of C/EBPβ expression appear to depend on Ins/IGF-1 signaling. Therefore, we propose that Rho-kinase and not CREB is a central target for PKA activation during the onset of the adipocyte differentiation program.
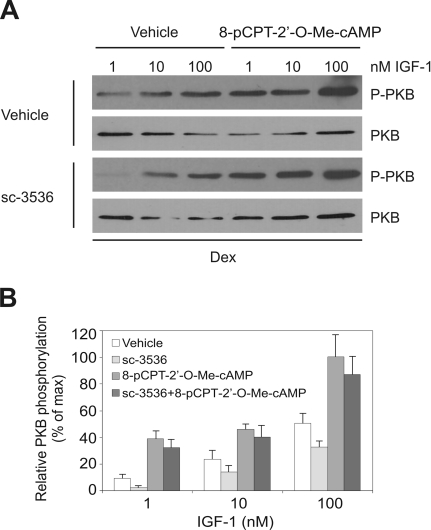
Ins/IGF-1 signaling is crucial for adipogenesis, so it is puzzling that inhibition of Rho-kinase is essential for induction of adipocyte differentiation since inhibition of Rho-kinase also impairs Ins signaling in adipocytes (27). We reasoned that activation of Epac might overcome the negative effect of Rho-kinase inhibition on Ins/IGF-1 signaling. In fact, Epac activation has been shown to potentiate Ins signaling in muscle cells (7). The supraphysiological concentration of Ins used in the standard Dex-Ins-IBMX differentiation protocols mimics IGF-1, the main adipogenic inducer, by interacting with the IGF-1 receptor (59, 61). To demonstrate that inhibition of Rho-kinase impaired IGF-1/Ins signaling in 3T3-L1 preadipocytes, the effect of the Rho-kinase-inhibitor sc-3536 was studied in cells stimulated with increasing concentrations of IGF-1 in the presence or absence of the Epac activator 8-pCPT-2′-O-Me-cAMP. The fetal calf serum used in the present experiments contained 10 nM IGF-1 (data not shown). Hence, the final concentration of IGF-1 in the medium containing 10% serum was 1 nM. Phosphorylation of PKB was determined as a marker of IGF-1 signaling. We found that the Rho-kinase inhibitor decreased IGF-1-dependent PKB phosphorylation. Importantly, activation of Epac enhanced PKB phosphorylation and restored the IGF-1-dependent PKB phosphorylation in the presence of the Rho-kinase inhibitor (Fig. 7). Thus, Epac activation stimulated IGF-1 signaling whether the Rho-kinase was inhibited or not.
FIG. 7.
Activation of Epac enhances Ins/IGF-1 signaling in 3T3-L1 cells (A) 3T3-L1 cells were grown to 2 days postconfluence and then treated with Dex and increasing concentrations of IGF-1 in the absence or presence of 10 μM sc-3536 and 200 μM 8-pCPT-2′-O-Me-cAMP, as indicated in the figure. After 15 min, whole-cell extracts were prepared and the levels of phosphorylated PKB and total PKB were determined by Western blotting. Shown is one representative experiment out of three independent experiments. (B) Quantification of the relative levels of PKB phosphorylation. Autoradiographs were analyzed by densitometric scanning, and the levels of phosphorylated PKB relative to total PKB were determined. The error bars represent standard deviations (n = 3).
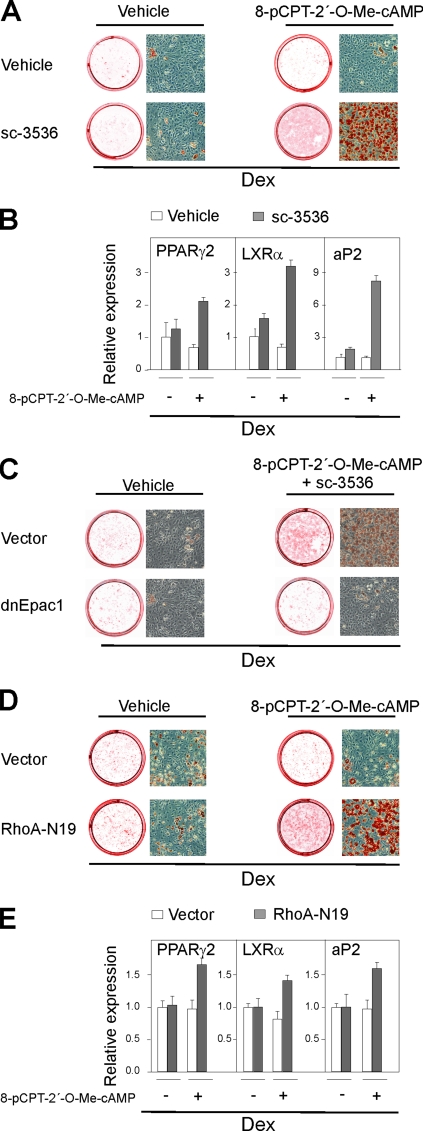
Based on the results above, we predicted that if the Rho-kinase was inhibited the activation of Epac could promote adipogenesis without PKA activation or addition of Ins/IGF-1 on top of the 1 nM basal level of IGF-1 in the medium. To test this prediction, we first treated 3T3-L1 preadipocytes with 8-pCPT-2′-O-Me-cAMP and the Rho-kinase inhibitor sc-3536 in the absence of added Ins/IGF-1 and IBMX. Pharmacological inhibition of the Rho-kinase by sc-3536 had no effect on adipogenesis, whether determined by oil red O staining or adipocyte marker gene expression when administered alone. Similarly, addition of the Epac activator 8-pCPT-2′-O-Me-cAMP alone had no effect on adipogenesis. However, inhibition of Rho-kinase dramatically enhanced differentiation when combined with the Epac activator 8-pCPT-2′-O-Me-cAMP (Fig. 8A and B). Expression of the dnEpac prevented, as expected, the 8-pCPT-2′-O-Me-cAMP-induced enhancement of adipocyte differentiation (Fig. 8C). To corroborate the results obtained by pharmacological inhibition of the Rho-kinase, we performed parallel experiments in which 3T3-L1 cells were transduced with either an empty retroviral vector or a retroviral vector expressing a dominant-negative version of RhoA (RhoA-N19). In agreement with the results obtained with the chemical Rho-kinase inhibitor sc-3536, activation of Epac induced adipocyte differentiation, as determined by oil red O staining and adipocyte marker gene expression in cells expressing the dominant-negative version of RhoA, but not in cells transduced with the empty vector (Fig. 8D and E). We conclude that Epac activation is essential for adipogenesis in cells exposed to physiological levels of Ins/IGF-1 when the Rho-kinase is inhibited.
FIG. 8.
Activation of Epac is sufficient to induce differentiation of 3T3-L1 cells when Rho-kinase is inhibited. 3T3-L1 cells were grown to 2 days postconfluence and induced to differentiate by Dex in the absence or presence of 10 μM sc-3536. Additionally, 200 μM 8-pCPT-2′-O-Me-cAMP was present from day 0 to day 2 as indicated. On day 8, the cells were stained with oil red O and photographed (A), total RNA was isolated, and the expression of PPARγ, LXRα, and aP2 was determined by RT-qPCR. The error bars represent standard deviations (n = 3) (B). 3T3-L1 cells were retrovirally transduced with an empty vector or a vector expressing a dominant negative form of Epac1 (dnEpac1). At 2-days post confluence they were induced to differentiate by Dex in the absence or presence of 10 μM sc-3536. Additionally, 200 μM 8-pCPT-2′-O-Me-cAMP was present from day 0 to day 2 as indicated. On day 8, the cells were stained with oil red O and photographed (C). 3T3-L1 cells were retrovirally transduced with an empty vector or a vector expressing a dominant-negative form of RhoA (RhoA-N19). At 2 days postconfluence, they were induced to differentiate by Dex in the absence or presence of 200 μM 8-pCPT-2′-O-Me-cAMP. On day 8, the cells were stained with oil red O and photographed. (D) Total RNA was isolated, and the expression of PPARγ, LXRα, and aP2 was determined by RT-qPCR. The error bars represent standard deviations (n = 3) (E).
DISCUSSION
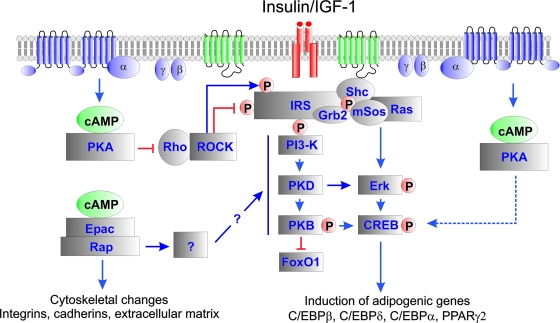
The differentiation of fibroblast-like cells into adipocytes involves dramatic changes of cell shape and function, which can be studied in vitro. In spite of a large number of studies pointing to the importance of elevated cAMP for initiation of adipocyte differentiation, the contribution of the two cAMP receptor families PKA and Epac has not been addressed. Neither has the interplay between cAMP and Rho and Rho-kinase. The present study shows that the effect of elevated cAMP levels depends on the concerted activation of both PKA and Epac1 to promote adipogenesis of both 3T3-L1 preadipocytes and mouse embryo fibroblasts. A model of such activation is shown in Fig. 9. The stimulation via Epac1 involves activation of Rap and is counteracted by dominant-negative Rap1, while the action of PKA involves inhibition of Rho and can be substituted for by dominant-negative Rho or inhibitors of Rho-kinase. These new findings require a reassessment of the role of cAMP in adipogenesis.
FIG. 9.
Model for the role of cAMP-stimulated adipogenesis via PKA- end Epac1-dependent processes. Increased levels of cAMP activate both PKA- and Epac-dependent pathways. Activation of PKA leads to repression of Rho-kinase activity by targeting either the Rho-kinase or the upstream regulator Rho. High levels of Rho kinase activity inhibit Ins/IGF-1-dependent signaling, and attenuation of Rho-kinase activity is crucial for adipogenesis. However, low levels of Rho-kinase activity also enhance Ins/IGF-1-dependent signaling, and PKA-mediated inhibition of the Rho-kinase impairs Ins/IGF-1-dependent signaling. This is counteracted by the simultaneous activation of an Epac1/Rap1-dependent pathway, which also induces important changes in cytoskeletal organization, adhesion, and extracellular matrix. Activation of CREB is not dependent on PKA activity, but rather requires ERK activity during the initial stages of adipogenesis.
In the context of adipocyte differentiation, PKA has previously been considered as the sole target of cAMP, whose differentiation-promoting effect has been ascribed to the activating phosphorylation of Ser-133 of CREB, an activator of the C/EBPβ promoter and pivotal regulator of adipocyte differentiation (26, 56, 57, 72). While our results do not question the importance of CREB-dependent activation of the adipogenic program, our results argue against PKA-mediated phosphorylation of CREB being indispensable for CREB activation during the initiation of adipocyte differentiation of 3T3-L1 preadipocytes using the standard Dex, Ins, and IBMX protocol. The selective PKA inhibitors Rp-8-Br-cAMPS/Rp-cAMPS did not diminish MDI-induced CREB phosphorylation. In contrast, the MEK inhibitor U0126 completely prevented CREB phosphorylation, underscoring the importance of signaling through the Ins/IGF-1-stimulated Ras/Raf/MEK/ERK pathway for phosphorylation of CREB (40). ERK1/2-dependent phosphorylation and activation of CREB and induction of C/EBPβ expression were also reported for leukemia inhibitory factor-induced adipocyte differentiation of Ob1771 and 3T3-F442A preadipocytes (4). It has been demonstrated that non-PKA-dependent phosphorylation of CREB may require additional cAMP-dependent signaling to activate CREB-mediated transcription (8, 12, 47). Our finding that adipocyte differentiation takes place and even is enhanced in the presence of the dual PKA and Rho kinase inhibitor H89 (see below for further discussion) indicates that if such cAMP-dependent activity is required, PKA is not directly involved. It remains to be established whether activation of the Epac-Rap branch of cAMP-dependent signaling may enhance CREB-mediated gene expression.
The first clue to the importance of Rho-kinase inhibition for the adipogenic action of PKA came through the observation that the PKA/Rho-kinase inhibitor H89 could substitute for PKA in supporting differentiation (Fig. 5A) (38, 51, 53). In the present report, we show in addition that a more specific Rho-kinase inhibitor as well as dominant-negative RhoA also could substitute for PKA and that PKA activation decreased active Rho-GTP and abolished the phosphorylation of the Rho-kinase target MLC. We propose therefore that the major role of PKA in promoting adipocyte differentiation may be through inhibition of Rho/Rho-kinase.
Mesenchymal determination and terminal differentiation along the adipocyte, myocyte, and osteocyte lineages are controlled by cell shape and cytoskeletal tension (34, 48, 50, 62), converging on regulation of Rho and Rho-kinase activity (48, 51, 63). We propose that PKA can decrease cell tension by converting RhoA to the inactive GTP-free form, which leads to relaxation of microfilaments, in part via inhibition of Rho-kinase-catalyzed phosphorylation of MLC. This relaxation of cell tension will lead to further inhibition of Rho. There are several known mechanisms for PKA-mediated inhibition of Rho. One is inactivating phosphorylation of RhoA by PKA (21, 41), a second is AKAP-Lbc phosphorylation by anchored PKA followed by recruitment of 14-3-3 and decreased Rho-GEF activity (20), and a third is PKA-catalyzed phosphorylation of Gα13 with consequent inhibition of Rho activation (1). Results presented here and previously published data show that repression of Rho activity is a point of convergence for adipogenesis-promoting signals. Sordella and coworkers have demonstrated that IGF-1-dependent phosphorylation of p190-B RhoGAP induces a translocation of p190-B RhoGAP to lipid rafts, leading to a down-regulation of Rho activity (63) Conversely, forced expression of the selective Rho-GEF, GEFT, prevents 3T3-L1 preadipocyte differentiation (9). Recently, it was furthermore shown that inhibition or genetic ablation of Rho-kinase (ROCK-II/ROKα) but not ROCK-I/ROKβ promoted adipocyte differentiation (51).
Epac was discovered as a cAMP-stimulated Rap activator (19), but has also been reported to act independently of Rap to stimulate the Jun-kinase (18) or activate R-ras (33, 39). In the 3T3-L1 model of adipocyte differentiation, we found that activation of Epac1 increases Rap1-GTP and that its stimulation of adipocyte differentiation was counteracted by dnRap1. This suggests that Epac1 acted to promote adipogenesis via activation of Rap1. A further clue to what role Epac1 might have in adipogenesis was provided by our observation that Epac1 activation could overcome the paradoxical negative effect of inhibition of Rho-kinase on IGF-1/Ins signaling. This may be related to the observations that inhibition of Rho-kinase activity impairs Ins signaling in cultured adipocytes and myocytes and causes Ins resistance with impaired glucose uptake in skeletal muscle of mice (27). Rho-kinase-dependent stimulation of Ins signaling was found to be associated with phosphorylation of serine residues 632 and 635 of Ins receptor substrate 1 (IRS-1) (27). On the other hand, Rho-kinase can inhibit Ins signaling through phosphorylation of serine 612 of IRS-1 (2, 3, 63). Thus, to achieve optimal insulin/IGF-1 signaling the activity of the Rho-kinase must be regulated within a very narrow window, a situation reminiscent of the p120 catenin-dependent regulation of adhesion junctions, where both inhibition of Rho activity and strong activation of Rho can block the formation of junctions (69). Thus, under conditions with elevated levels of cAMP resulting in PKA-dependent inhibition of Rho activity, effective Ins/IGF-1 signaling might dependent on a compensatory sensitizing effect by the Epac branch of the cAMP effector machinery.
Using phosphorylation of PKB as a measure of IGF-1 signaling, we demonstrate that Rho-kinase inhibition impaired and Epac1 activation enhanced IGF-1-dependent activation of PKB and, furthermore, we show that activation of Epac1 fully restored or even enhanced PKB activation in the presence of a Rho-kinase inhibitor. Similarly, activation of Epac1 potentiated Ins-dependent activation of PKB in skeletal muscle (7).
We speculate that activation of Rap may be one common feature of Epac and IGF-1 signaling, explaining at least in part the ability of Epac activation to compensate for limiting levels of Ins/IGF-1. Thus, the adaptor protein c-Crk, which recruits C3G, a GEF for Rap1, has been shown to be required as an early signaling mediator of IGF-1-induced adipocyte differentiation of 3T3-L1 preadipocytes (35). C3G is able to activate Rap1 in NIH 3T3 cells (43), and hence, it is conceivable that the same is true for 3T3-L1 adipocytes. How Epac-Rap1 activation sensitizes Ins/IGF-1 signaling in preadipocytes remains to be elucidated. In muscle, it was shown that activation of Epac promoted Ins-stimulated recruitment of phosphatidylinositol 3-kinase (PI3-K) to signaling complexes. However, no interaction between the p85 subunit of the PI3-K and Rap1 analogous to the interaction between cdc42 and p85 (68) could be detected (7), and the molecular basis for the increased PI3-K recruitment remains to be established.
The synergistic action of Ins/IGF-1 and cAMP signaling during initiation of adipocyte differentiation is remarkable and contrasts the normal interplay between insulin and cAMP signaling in liver, muscle, and mature adipocytes. In mature adipocytes, stimulation of β-adrenergic receptors increases cAMP levels, leading to PKA activation and stimulation of lipolysis by PKA-mediated phosphorylation of hormone-sensitive lipase and perilipin. In this setting, Ins disrupts the coupling between the β2-adrenergic receptor and PKA presumably through modulation of AKAP scaffolding proteins (71). Similarly, the channeling of cAMP signaling via Epac also seems to vary between preadipocytes and adipocytes. In preadipocytes, our results show that activation of Epac increases Ins/IGF-1 signaling and PKB activation, whereas activation of Epac by 8-pCPT-2′-O-Me-cAMP in primary rat adipocytes decreases Ins-dependent activation of PKB (73). Thus, cAMP-dependent signals appear to follow different routes in preadipocytes and mature adipocytes. In addition, the marked differences in the number of receptors for Ins and IGF-1, respectively, in preadipocytes and mature adipocytes (61) coupled with the increased expression of small G-proteins such as TC10α, which is recruited to CAP-Cbl-C3G complexes in lipid rafts in response to Ins and required for Ins non-PI3-K-dependent translocation of Glut4 (11, 13), may well in a competitive manner affect Epac-independent Rap activation. Further deciphering of the cross talk between cAMP-dependent signaling and Ins/IGF-1 signaling promises novel avenues for insight into the complex signaling governing the transition from preadipocytes and adipocytes as well as metabolic regulation in mature adipocytes.
Acknowledgments
We thank Johannes Bos, Ormond MacDougald, Eva Pålsson-McDermott, R Regazzi, and Reidun Kopperud for valuable plasmids.
This work was carried out as a part of the research program of the Danish Obesity Research Centre (DanORC). DanORC is supported by The Danish Council for Strategic Research (grant no. 2101-06-0005). This work was in addition supported by the Danish Natural Science Research Council, the Norwegian Research Council, and the NOVO Foundation.
L.M. and K.K. are founders and members of the board of BioLigands ApS, and R.K.P. is an employee of BioLigands ApS.
Footnotes
Published ahead of print on 7 April 2008.
REFERENCES
- 1.Baisamy, L., N. Jurisch, and D. Diviani. 2005. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J. Biol. Chem. 28015405-15412. [DOI] [PubMed] [Google Scholar]
- 2.Begum, N., O. A. Sandu, and N. Duddy. 2002. Negative regulation of rho signaling by insulin and its impact on actin on cytoskeleton organization in vascular smooth muscle cells: role of nitric oxide and cyclic guanosine monophosphate signaling pathways. Diabetes 512256-2263. [DOI] [PubMed] [Google Scholar]
- 3.Begum, N., O. A. Sandu, M. Ito, S. M. Lohmann, and A. Smolenski. 2002. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J. Biol. Chem. 2776214-6222. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte, N., B. W. Phillips, F. Massiera, P. Villageois, B. Wdziekonski, P. Saint-Marc, J. Nichols, J. Aubert, K. Saeki, A. Yuo, S. Narumiya, G. Ailhaud, and C. Dani. 2001. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins β and -δ in preadipocytes. Mol. Endocrinol. 152037-2049. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, C. N., S. E. Ross, K. A. Longo, L. Bajnok, N. Hemati, K. W. Johnson, S. D. Harrison, and O. A. MacDougald. 2002. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 27730998-31004. [DOI] [PubMed] [Google Scholar]
- 6.Bos, J. L. 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31680-686. [DOI] [PubMed] [Google Scholar]
- 7.Brennesvik, E. O., C. Ktori, J. Ruzzin, E. Jebens, P. R. Shepherd, and J. Jensen. 2005. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell. Signal. 171551-1559. [DOI] [PubMed] [Google Scholar]
- 8.Brindle, P., T. Nakajima, and M. Montminy. 1995. Multiple protein kinase A-regulated events are required for transcriptional induction by cAMP. Proc. Natl. Acad. Sci. USA 9210521-10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan, B. A., D. C. Mitchell, L. Zhao, W. Ma, L. J. Stafford, B.-B. Teng, and M. Liu. 2005. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol. Cell. Biol. 2511089-11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, Z., R. Umek, and S. McKnight. 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 51538-1552. [DOI] [PubMed] [Google Scholar]
- 11.Chang, L., S. H. Chiang, and A. R. Saltiel. 2007. TC10alpha is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology 14827-33. [DOI] [PubMed] [Google Scholar]
- 12.Chawla, S., and H. Bading. 2001. CREB/CBP and SRE-interacting transcriptional regulators are fast on-off switches: duration of calcium transients specifies the magnitude of transcriptional responses. J. Neurochem. 79849-858. [DOI] [PubMed] [Google Scholar]
- 13.Chiang, S. H., C. A. Baumann, M. Kanzaki, D. C. Thurmond, R. T. Watson, C. L. Neudauer, I. G. Macara, J. E. Pessin, and A. R. Saltiel. 2001. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410944-948. [DOI] [PubMed] [Google Scholar]
- 14.Christensen, A. E., and S. O. Doskeland. 2003. Cyclic nucleotide analogs, p. 549-554. In R. Bradshaw and E. Dennis (ed.), Handbook of cell signaling, vol. 2. Academic Press, San Diego, CA. [Google Scholar]
- 15.Christensen, A. E., F. Selheim, J. de Rooij, S. Dremier, F. Schwede, K. K. Dao, A. Martinez, C. Maenhaut, J. L. Bos, H. G. Genieser, and S. O. Doskeland. 2003. cAMP analog mapping of Epac1 and cAMP kinase: discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J. Biol. Chem. 27835394-35402. [DOI] [PubMed] [Google Scholar]
- 16.Christy, R. J., K. H. Kaestner, D. E. Geiman, and M. D. Lane. 1991. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 882593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullere, X., S. K. Shaw, L. Andersson, J. Hirahashi, F. W. Luscinskas, and T. N. Mayadas. 2004. Regulation of vascular endothelial barrier function by Epac, a cAMP activated exchange factor for Rap GTPase. Blood 1051950-1955. [DOI] [PubMed] [Google Scholar]
- 18.De Jesus, M. L., M. B. Stope, P. A. O. Weernink, Y. Mahlke, C. Borgermann, V. N. Ananaba, C. Rimmbach, D. Rosskopf, M. C. Michel, K. H. Jakobs, and M. Schmidt. 2006. Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J. Biol. Chem. 28121837-21847. [DOI] [PubMed] [Google Scholar]
- 19.de Rooij, J., F. J. T. Zwartkruis, M. H. G. Verheijen, R. H. Cool, S. M. B. Nijman, A. Wittinghofer, and J. L. Bos. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396474-477. [DOI] [PubMed] [Google Scholar]
- 20.Diviani, D., L. Abuin, S. Cotecca, and L. Pansier. 2004. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 232811-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong, J. M., T. Leung, E. Manser, and L. Lim. 1998. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J. Biol. Chem. 27322554-22562. [DOI] [PubMed] [Google Scholar]
- 22.Ekanger, R., and S. O. Døskeland. 1988. Use of antibody-sepharose columns to study hormonal activation of cAMP-dependent protein kinase isozymes. Methods Enzymol. 15997-104. [DOI] [PubMed] [Google Scholar]
- 23.Enserink, J. M., A. E. Christensen, J. de Rooij, M. van Triest, F. Schwede, H. G. Genieser, S. O. Doskeland, J. L. Blank, and J. L. Bos. 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4901-906. [DOI] [PubMed] [Google Scholar]
- 24.Enserink, J. M., L. S. Price, T. Methi, M. Mahic, A. Sonnenberg, J. L. Bos, and K. Tasken. 2004. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the α3β1 integrin but not the α6β4 integrin. J. Biol. Chem. 27944889-44896. [DOI] [PubMed] [Google Scholar]
- 25.Farmer, S. R. 2006. Transcriptional control of adipocyte formation. Cell Metab. 4263-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox, K. E., D. M. Fankell, P. F. Erickson, S. M. Majka, J. T. Crossno, Jr., and D. J. Klemm. 2006. Depletion of cAMP-response element binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer binding protein (C/EBP) alpha, C/EBP beta or PPAR gamma 2. J. Biol. Chem. 28140341-40353. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa, N., P. Ongusaha, W. J. Jahng, K. Araki, C. S. Choi, H. J. Kim, Y. H. Lee, K. Kaibuchi, B. B. Kahn, H. Masuzaki, J. K. Kim, S. W. Lee, and Y. B. Kim. 2005. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2119-129. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez, G., and M. Montminy. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59675-680. [DOI] [PubMed] [Google Scholar]
- 29.Hamm, J. K., B. H. Park, and S. R. Farmer. 2001. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 27618464-18471. [DOI] [PubMed] [Google Scholar]
- 30.Hansen, J. B., and K. Kristiansen. 2006. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem. J. 398153-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen, J. B., R. K. Petersen, C. Jorgensen, and K. Kristiansen. 2002. Deregulated MAPK activity prevents adipocyte differentiation of fibroblasts lacking the retinoblastoma protein. J. Biol. Chem. 27726335-26339. [DOI] [PubMed] [Google Scholar]
- 32.Hansen, J. B., H. Zhang, T. H. Rasmussen, R. K. Petersen, E. N. Flindt, and K. Kristiansen. 2001. Peroxisome proliferator-activated receptor delta (PPARdelta)-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J. Biol. Chem. 2763175-3182. [DOI] [PubMed] [Google Scholar]
- 33.Hochbaum, D., T. Tanos, F. Ribeiro-Neto, D. Altschuler, and O. A. Coso. 2003. Activation of JNK by Epac is independent of its activity as a Rap guanine nucleotide exchanger. J. Biol. Chem. 27833738-33746. [DOI] [PubMed] [Google Scholar]
- 34.Jakkaraju, S., Z. Zhe, D. Pan, R. Choudhury, and L. Schuger. 2005. TIPs are tension-responsive proteins involved in myogenic versus adipogenic differentiation. Dev. Cell 939-49. [DOI] [PubMed] [Google Scholar]
- 35.Jin, S., B. Zhai, Z. Qiu, J. Wu, M. D. Lane, and K. Liao. 2000. c-Crk, a substrate of the insulin-like growth factor-1 receptor tyrosine kinase, function as an early signal mediator in the adipocyte differentiation process. J. Biol. Chem. 27534344-34352. [DOI] [PubMed] [Google Scholar]
- 36.Kang, G., O. G. Chepurny, and G. G. Holz. 2001. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J. Physiol. (London) 536375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang, G., J. W. Joseph, O. G. Chepurny, M. Monaco, M. B. Wheeler, J. L. Bos, F. Schwede, H. G. Genieser, and G. G. Holz. 2003. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J. Biol. Chem. 2788279-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato, Y., N. Ozaki, T. Yamada, Y. Miura, and Y. Oiso. 2007. H-89 potentiates adipogenesis in 3T3-L1 cells by activating insulin signaling independently of protein kinase A. Life Sci. 80476-483. [DOI] [PubMed] [Google Scholar]
- 39.Keiper, M., M. B. Stope, D. Szatkowski, A. Bohm, K. Tysack, F. vom Dorp, O. Saur, P. A. Oude Weernink, S. Evellin, K. H. Jakobs, and M. Schmidt. 2004. Epac- and Ca2+-controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J. Biol. Chem. 27946497-46508. [DOI] [PubMed] [Google Scholar]
- 40.Klemm, D. J., W. J. Roesler, T. Boras, L. A. Colton, K. Felder, and J. E. B. Reusch. 1998. Insulin stimulates cAMP-response element binding protein activity in HepG2 and 3T3-L1 cell lines. J. Biol. Chem. 273917-923. [DOI] [PubMed] [Google Scholar]
- 41.Lang, P., F. Gesbert, M. Delespine-Carmagnat, R. Stancou, M. Pouchelet, and J. Bertoglio. 1996. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 15510-519. [PMC free article] [PubMed] [Google Scholar]
- 42.Leemhuis, J., S. Boutillier, G. Schmidt, and D. K. Meyer. 2002. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J. Pharmacol. Exp. Ther. 3001000-1007. [DOI] [PubMed] [Google Scholar]
- 43.Ling, L., T. Zhu, and P. E. Lobie. 2003. Src-CrkII-C3G-dependent activation of Rap1 switches growth hormone-stimulated p44/42 MAP kinase and JNK/SAPK activities. J. Biol. Chem. 27827301-27311. [DOI] [PubMed] [Google Scholar]
- 44.Lukas, J., J. Bartkova, M. Rohde, M. Strauss, and J. Bartek. 1995. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol. Cell. Biol. 152600-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDougald, O. A., and M. D. Lane. 1995. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 64345-373. [DOI] [PubMed] [Google Scholar]
- 46.Madsen, L., R. K. Petersen, M. B. Sørensen, C. Jørgensen, P. Hallenborg, L. Pridal, J. Fleckner, E.-Z. Amri, P. Krieg, G. Furstenberger, R. K. Berge, and K. Kristiansen. 2003. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. Biochem. J. 375539-549. [DOI] [PubMed] [Google Scholar]
- 47.Mayr, B. M., G. Canettieri, and M. R. Montminy. 2001. Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc. Natl. Acad. Sci. USA 9810936-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McBeath, R., D. M. Pirone, C. M. Nelson, K. Bhadriraju, and C. S. Chen. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6483-495. [DOI] [PubMed] [Google Scholar]
- 49.Mei, F. C., J. Qiao, O. M. Tsygankova, J. L. Meinkoth, L. A. Quilliam, and X. Cheng. 2002. Differential signaling of cyclic AMP. J. Biol. Chem. 27711497-11504. [DOI] [PubMed] [Google Scholar]
- 50.Meyers, V. E., M. Zayzafoon, J. T. Douglas, and J. M. McDonald. 2005. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Miner. Res. 201858-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noguchi, M., K. Hosoda, J. Fujikura, M. Fujimoto, H. Iwakura, T. Tomita, T. Ishii, N. Arai, M. Hirata, K. Ebihara, H. Masuzaki, H. Itoh, S. Narumiya, and K. Nakao. 2007. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J. Biol. Chem. 28229574-29583. [DOI] [PubMed] [Google Scholar]
- 52.Ozaki, N., T. Shibasaki, Y. Kashima, T. Miki, K. Takahashi, H. Ueno, Y. Sunaga, H. Yano, Y. Matsuura, T. Iwanaga, Y. Takai, and S. Seino. 2000. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat. Cell Biol. 2805-811. [DOI] [PubMed] [Google Scholar]
- 53.Petersen, R. K., C. Jorgensen, A. C. Rustan, L. Froyland, K. Muller-Decker, G. Furstenberger, R. K. Berge, K. Kristiansen, and L. Madsen. 2003. Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases. J. Lipid Res. 442320-2330. [DOI] [PubMed] [Google Scholar]
- 54.Rangarajan, S., J. M. Enserink, H. B. Kuiperij, J. de Rooij, L. S. Price, F. Schwede, and J. L. Bos. 2003. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J. Cell Biol. 160487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reedquist, K. A., E. Ross, E. A. Koop, R. M. Wolthuis, F. J. Zwartkruis, Y. van Kooyk, M. Salmon, C. D. Buckley, and J. L. Bos. 2000. The small GTPase, Rap1, mediates CD31-induced integrin adhension. J. Cell Biol. 1481151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reusch, J. E. B., L. A. Colton, and D. J. Klemm. 2000. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 201008-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reusch, J. E. B., and D. J. Klemm. 2002. Inhibition of cAMP-response element-binding protein activity decreases protein kinase B/Akt expression in 3T3-L1 adipocytes and induces apoptosis. J. Biol. Chem. 2771426-1432. [DOI] [PubMed] [Google Scholar]
- 58.Rosen, E. D., and O. A. MacDougald. 2006. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7885-896. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt, W., G. Poll-Jordan, and G. Loffler. 1990. Adipose conversion of 3T3-L1 cells in a serum-free culture system depends on epidermal growth factor, insulin-like growth factor I, corticosterone, and cyclic AMP. J. Biol. Chem. 26515489-15495. [PubMed] [Google Scholar]
- 60.Shi, G.-X., H. Rehmann, and D. A. Andres. 2006. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol. Cell. Biol. 269136-9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith, P. J., L. S. Wise, R. Berkowitz, C. Wan, and C. S. Rubin. 1988. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 2639402-9408. [PubMed] [Google Scholar]
- 62.Sordella, R., M. Classon, K. Hu, SF. Matheson, M. Brouns, B. Fine, L. Zhang, S. Takase, Y. Yamada, and J. Settleman. 2002. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev. Cell 2553-565. [DOI] [PubMed] [Google Scholar]
- 63.Sordella, R., W. Jiang, G. C. Chen, M. Curto, and J. Settleman. 2003. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113147-158. [DOI] [PubMed] [Google Scholar]
- 64.Tang, Q.-Q., M.-S. Jiang, and M. D. Lane. 1999. Repressive effect of Sp1 on the C/EBPα gene promoter: role in adipocyte differentiation. Mol. Cell. Biol. 194855-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang, Q. Q., and M. D. Lane. 1999. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 132231-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang, Q. Q., J. W. Zhang, and M. D. Lane. 2004. Sequential gene promoter interactions by C/EBPβ, C/EBPα, and PPARγ during adipogenesis. Biochem. Biophys. Res. Commun. 318213-218. [DOI] [PubMed] [Google Scholar]
- 67.Tzameli, I., H. Fang, M. Ollero, H. Shi, J. K. Hamm, P. Kievit, A. N. Hollenberg, and J. S. Flier. 2004. Regulated production of a peroxisome proliferator-activated receptor-γ ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J. Biol. Chem. 27936093-36102. [DOI] [PubMed] [Google Scholar]
- 68.Usui, I., T. Imamura, J. Huang, H. Satoh, and J. M. Olefsky. 2003. Cdc42 is a Rho GTPase family member that can mediate insulin signaling to glucose transport in 3T3-L1 adipocytes. J. Biol. Chem. 27813765-13774. [DOI] [PubMed] [Google Scholar]
- 69.Wildenberg, G. A., M. R. Dohn, R. H. Carnahan, M. A. Davis, N. A. Lobdell, J. Settlemen, and A. B. Reynolds. 2007. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 1271027-1039. [DOI] [PubMed] [Google Scholar]
- 70.Yeh, W., Z. Cao, M. Classon, and S. McKnight. 1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 9168-181. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, J., C. J. Hupfeld, S. S. Taylor, J. M. Olefsky, and R. Y. Tsien. 2005. Insulin disrupts β-adrenergic signalling to protein kinase A in adipocytes. Nature 437569-573. (Letter.) [DOI] [PubMed] [Google Scholar]
- 72.Zhang, J.-W., D. J. Klemm, C. Vinson, and M. D. Lane. 2004. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J. Biol. Chem. 2794471-4478. [DOI] [PubMed] [Google Scholar]
- 73.Zmuda-Trzebiatowska, E., V. Manganiello, and E. Degerman. 2007. Novel mechanisms of the regulation of protein kinase B in adipocytes; implications for protein kinase A, Epac, phosphodiesterases 3 and 4. Cell. Signal. 1981-86. [DOI] [PubMed] [Google Scholar]