Abstract
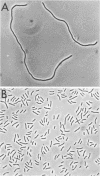
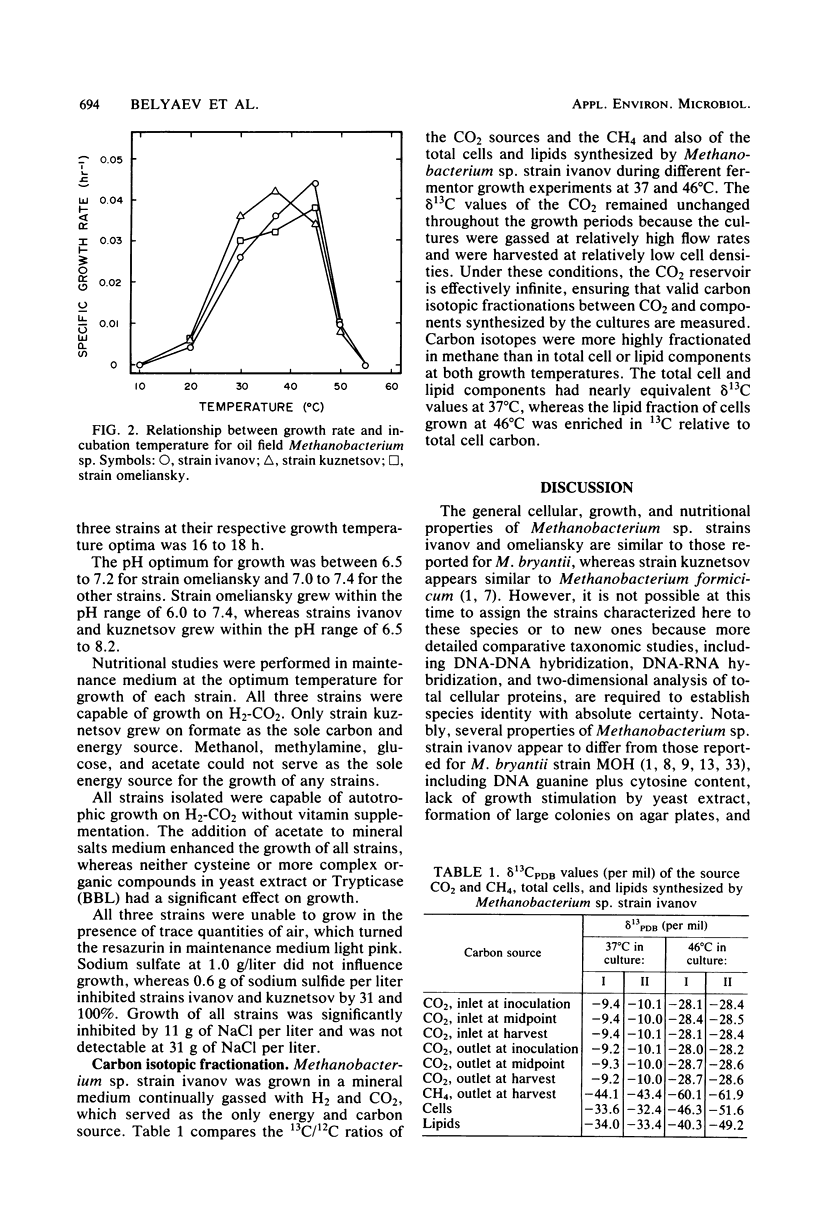
Selective enrichment culture techniques were employed to obtain mixed cultures of methanogenic rods and sarcina from surface flooding waters and deep subsurface (∼1650 m) oil-bearing sedimentary rocks and formation waters sampled from an old oil field in the U.S.S.R. previously reported to display active biological methanogenesis. The methanogens were selectively isolated as colonies on agar petri dishes that were incubated in a novel container. The general cellular and growth features of three Methanobacterium isolates were determined. These strains grew optimally at 37 to 45°C in anaerobic pressure tube cultures with a doubling time of 16 to 18 h on H2-CO2 and proliferated as autotrophs. Acetate addition significantly enhanced the final cell yield. Growth of these strains was completely inhibited by either 0.6 g of sodium sulfide per liter or 31.0 of sodium chloride per liter, but growth was not inhibited by either 0.3 g of sodium sulfide per liter or 1.0 g of sodium sulfate per liter. One novel isolate, Methanobacterium sp. strain ivanov, was grown on H2-CO2, and the stable-carbon isotopic fractionations that occurred during synthesis of methane, cell carbon, and lipids were determined. The results of this study were used to examine the anomalous relationship between the isotopic and chemical compositions of natural gas occurring in the deep subsurface environment of the oil field.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNiro M. J., Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977 Jul 15;197(4300):261–263. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- Godsy E. M. Isolation of Methanobacterium bryantii from a Deep Aquifer by Using a Novel Broth-Antibiotic Disk Method. Appl Environ Microbiol. 1980 May;39(5):1074–1075. doi: 10.1128/aem.39.5.1074-1075.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. W., King R. E., Unger P. D., Phillips T. D., Hatkin J., Bowen J. H. Aflatoxicosis in swine. J Am Vet Med Assoc. 1978 Jun 1;172(11):1295–1297. [PubMed] [Google Scholar]
- Mink R. W., Dugan P. R. Tentative identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 Mar;33(3):713–717. doi: 10.1128/aem.33.3.713-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate assimilation pathway of Methanosarcina barkeri. J Bacteriol. 1979 Jan;137(1):332–339. doi: 10.1128/jb.137.1.332-339.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Anaerobic metabolism of immediate methane precursors in Lake Mendota. Appl Environ Microbiol. 1979 Feb;37(2):244–253. doi: 10.1128/aem.37.2.244-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]