Abstract
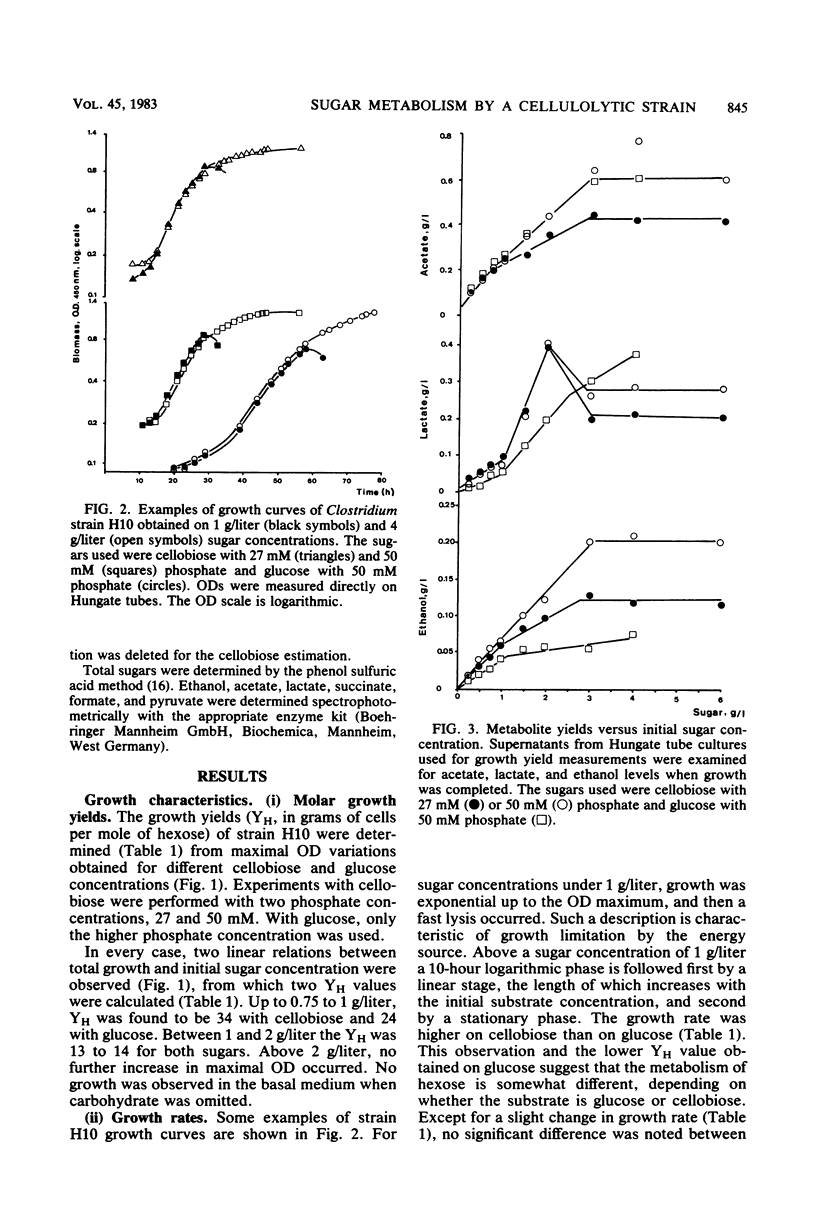
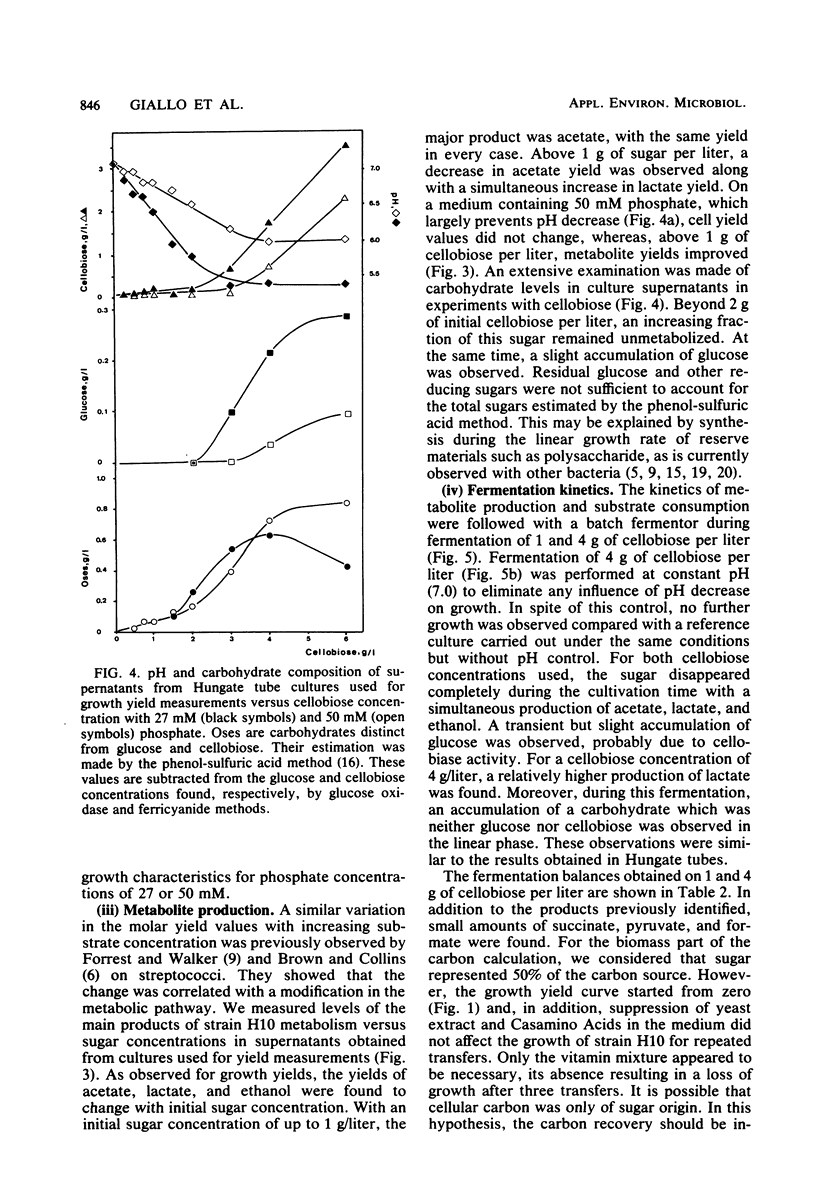
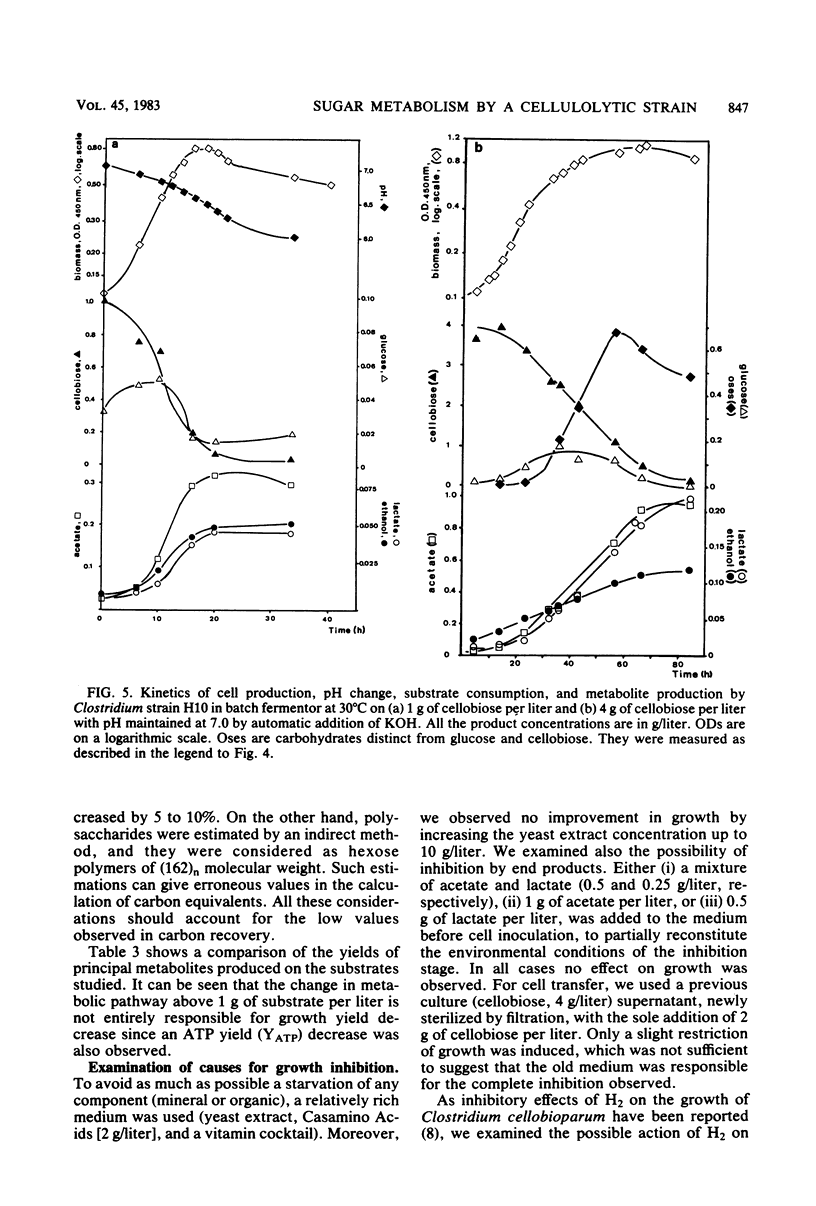
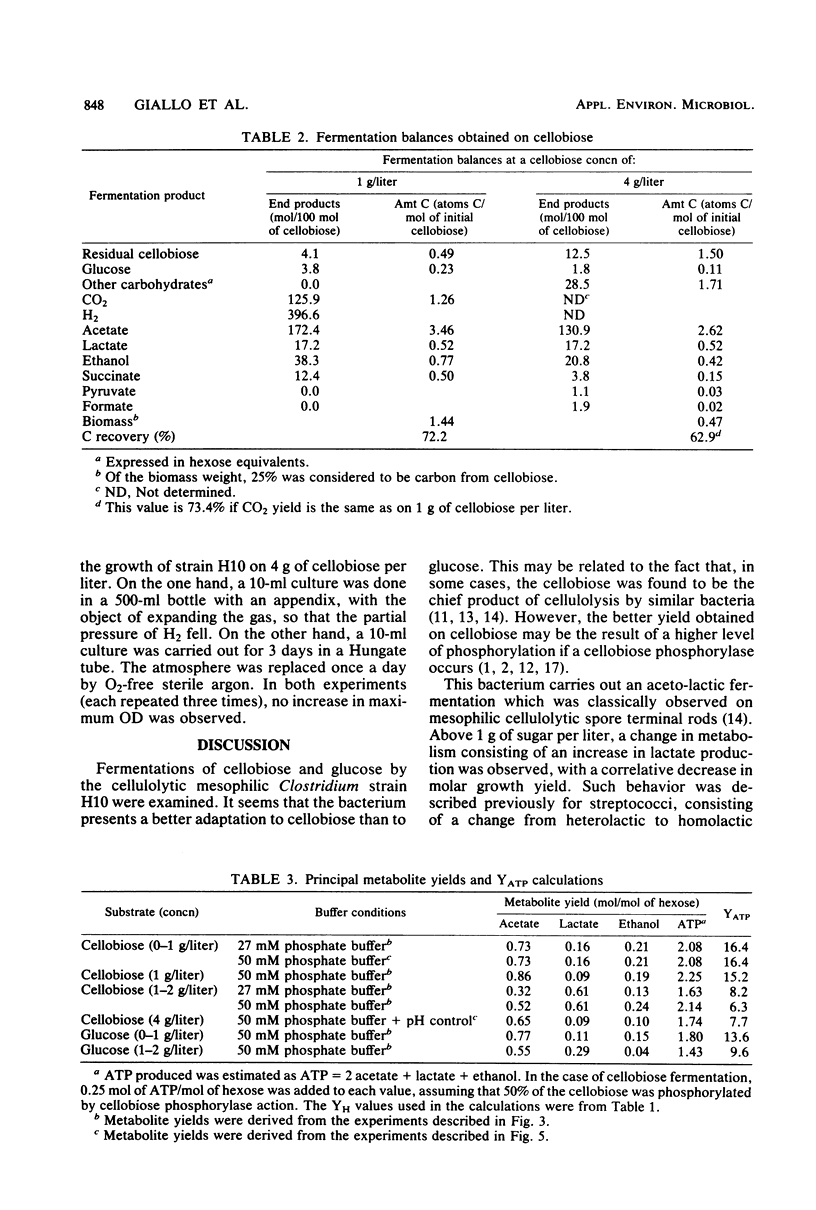
The metabolism of strain H10, a cellulolytic mesophilic Clostridium sp., was studied on glucose and cellobiose as energy and carbon sources. The main products of fermentation of both sugars were acetate, lactate, and ethanol. At low sugar levels, molar growth yields were better for cellobiose than for glucose. In both cases, an inhibition of growth was observed between 1 and 2 g/liter and a total inhibition after the latter concentration. Inhibition was not the result of low pH due to acid formation; growth under static pH conditions was limited in the same way. On the other hand, acetate and lactate had no inhibitory effect when added at concentrations equal to the final titers. Concomitant with the inhibition of growth was a change in metabolic pathways for sugar concentrations between 1 and 2 g/liter, i.e., the production of lactate was higher. After complete inhibition of growth, an accumulation of carbohydrates which were neither glucose nor cellobiose was observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYERS W. A. Phosphorylation of cellobiose and glucose by Ruminococcus flavefaciens. J Bacteriol. 1958 Nov;76(5):515–517. doi: 10.1128/jb.76.5.515-517.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. K. Purification and specificity of cellobiose phosphorylase from Clostridium thermocellum. J Biol Chem. 1968 Jun 10;243(11):2899–2904. [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Collins E. B. End products and fermentation balances for lactic streptococci grown aerobically on low concentrations of glucose. Appl Environ Microbiol. 1977 Jan;33(1):38–42. doi: 10.1128/aem.33.1.38-42.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Chung K. T. Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl Environ Microbiol. 1976 Mar;31(3):342–348. doi: 10.1128/aem.31.3.342-348.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORREST W. W., WALKER D. J. SYNTHESIS OF RESERVE MATERIALS FOR ENDOGENOUS METABOLISM IN STREPTOCOCCUS FAECALIS. J Bacteriol. 1965 Jun;89:1448–1452. doi: 10.1128/jb.89.6.1448-1452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULCHER F. H., KING K. W. Disaccharide preference of an aerobic cellulolytic bacterium, Cellvibrio gilvus n. sp. J Bacteriol. 1958 Dec;76(6):565–570. doi: 10.1128/jb.76.6.565-570.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULCHER F. H., KING K. W. Metabolic basis for disaccharide preference in a Cellvibrio. J Bacteriol. 1958 Dec;76(6):571–577. doi: 10.1128/jb.76.6.571-577.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: I. The Culture and Physiology of an Anaerobic Cellulose-digesting Bacterium. J Bacteriol. 1944 Nov;48(5):499–513. doi: 10.1128/jb.48.5.499-513.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray C., Nicoli J., Audiffren P. Microdosage colorimetrique des oses neutres par l'acide sulfurique et le phénol. Influence des sels et des proteines. Med Trop (Mars) 1966 Jul-Aug;26(4):382–390. [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Differential metabolism of cellobiose and glucose by Clostridium thermocellum and Clostridium thermohydrosulfuricum. J Bacteriol. 1982 Jun;150(3):1391–1399. doi: 10.1128/jb.150.3.1391-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977 Feb;33(2):289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]


