Abstract
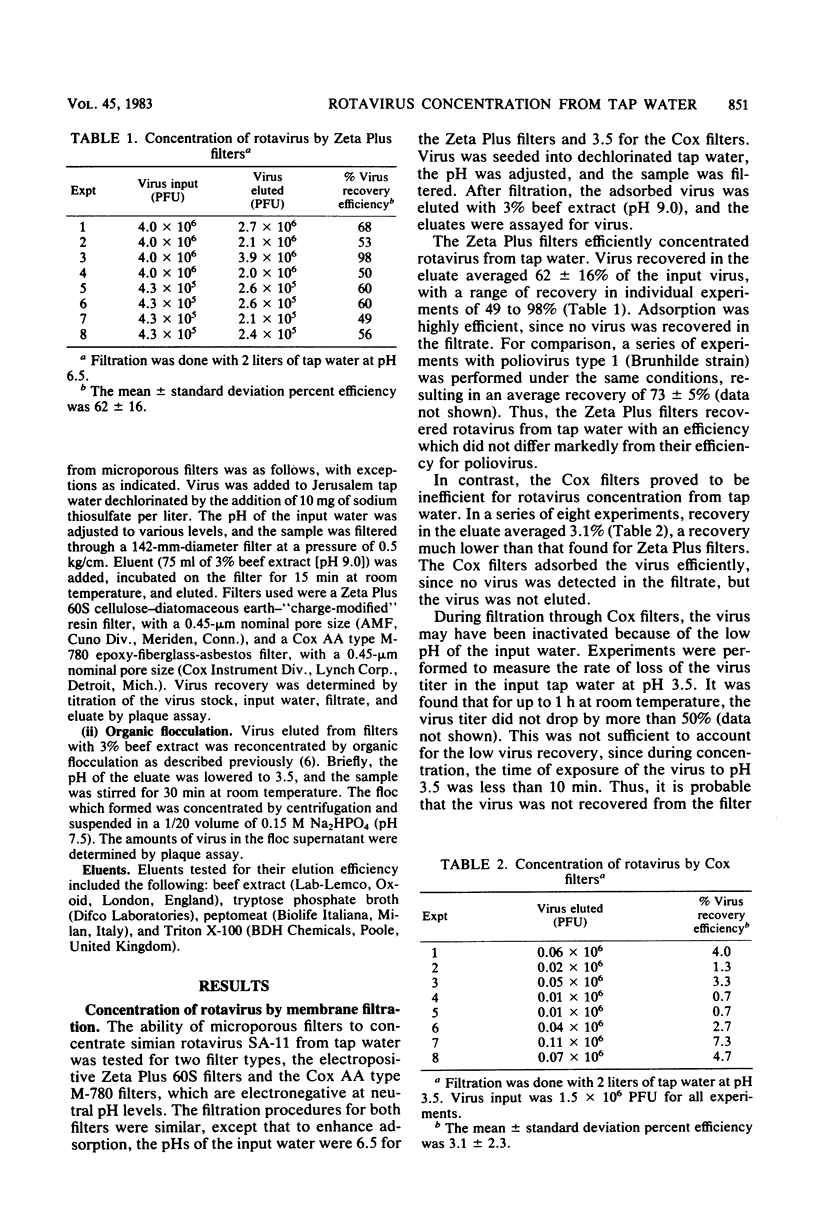
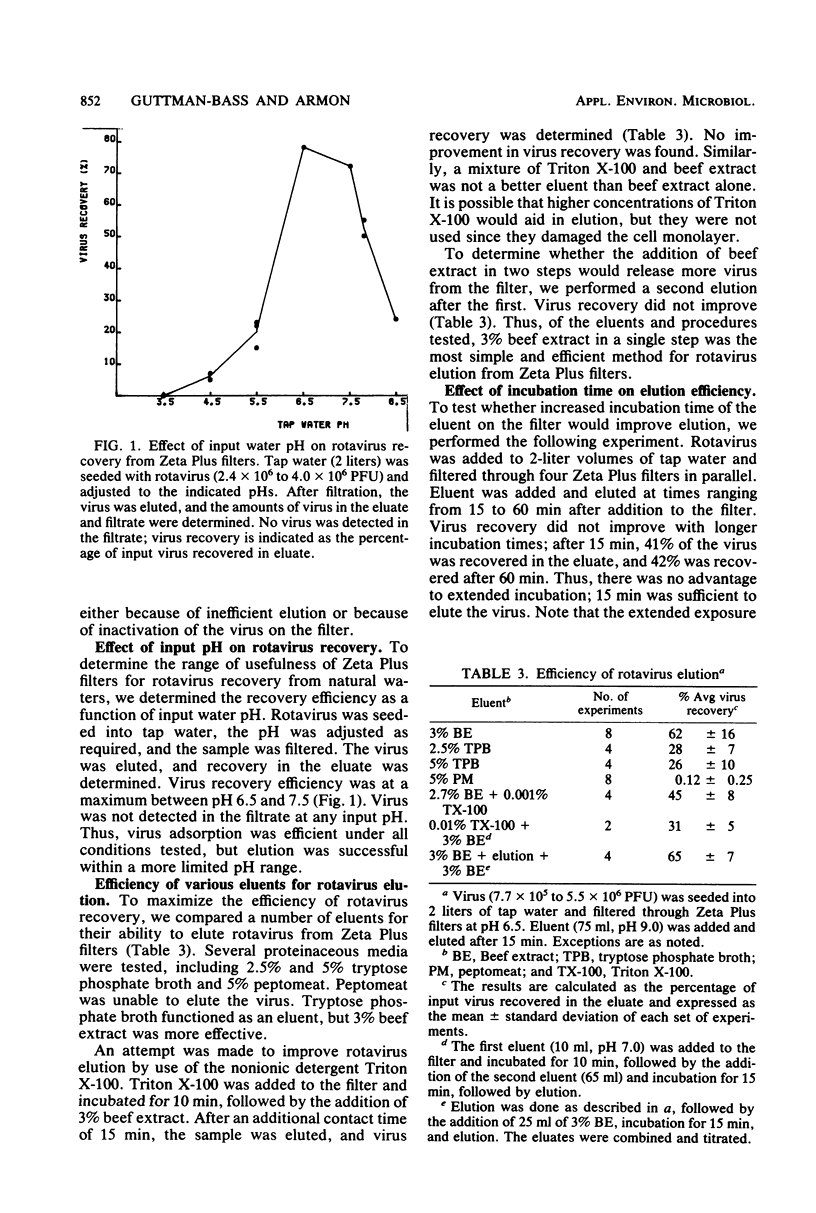
Simian rotavirus SA-11 was concentrated from tap water by adsorption to and elution from microporous filters, followed by organic flocculation. Two types of filters were compared for their ability to concentrate the virus. Both Zeta Plus 60S and Cox AA type M-780 filters were efficient for virus adsorption, but the efficiency of virus elution was higher with Zeta Plus than with Cox filters. Optimum conditions for virus recovery from Zeta Plus filters included an input water pH of 6.5 to 7.5 and the use of 3% beef extract (pH 9.0) for elution. Under these conditions, an average of 62 to 100% of the virus was recovered in the concentrate. Organic flocculation was used as a second-step concentration method, with average recoveries of 47 to 69%. When the two methods were used to concentrate small numbers (7 to 75 PFU/liter) of input rotavirus, an average of 75 ± 40% recovery was achieved. With large volumes of input water, however, recovery was reduced to 16 ± 7%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Smith E. M. Comparison between adsorption of poliovirus and rotavirus by aluminum hydroxide and activated sludge flocs. Appl Environ Microbiol. 1978 Feb;35(2):360–363. doi: 10.1128/aem.35.2.360-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Wallis C., Melnick J. L., DuPont H. L. A simple method for concentration of enteroviruses and rotaviruses from cell culture harvests using membrane filters. Intervirology. 1978;9(1):56–59. doi: 10.1159/000148921. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E. F., Vaughn J. M., Thomas M. Z., Vicale T. J. Efficiency of beef extract for the recovery of poliovirus from wastewater effluents. Appl Environ Microbiol. 1978 Oct;36(4):544–548. doi: 10.1128/aem.36.4.544-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramia S., Sattar S. A. Concentration of seeded simian rotavirus SA-11 from potable waters by using talc-celite layers and hydroextraction. Appl Environ Microbiol. 1980 Mar;39(3):493–499. doi: 10.1128/aem.39.3.493-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramia S., Sattar S. A. Rotavirus concentration from cell culture harvests: trypsin treatment followed by hydroextraction. Appl Environ Microbiol. 1980 Dec;40(6):1133–1135. doi: 10.1128/aem.40.6.1133-1135.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoub B. D., Lecatsas G., Prozesky O. W. Antigenic relationship between human and simian rotaviruses. J Med Microbiol. 1977 Feb;10(1):1–6. doi: 10.1099/00222615-10-1-1. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Estes M. K., Graham D. Y., Gerba C. P. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979 Jun;43(3):513–519. doi: 10.1099/0022-1317-43-3-513. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P. Development of a method for detection of human rotavirus in water and sewage. Appl Environ Microbiol. 1982 Jun;43(6):1440–1450. doi: 10.1128/aem.43.6.1440-1450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Glass J. S. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980 Aug;40(2):201–210. doi: 10.1128/aem.40.2.201-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Jones B. L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl Environ Microbiol. 1979 Mar;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann J. Detection of rotavirus in sewage. Appl Environ Microbiol. 1981 Apr;41(4):1043–1045. doi: 10.1128/aem.41.4.1043-1045.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


