Abstract
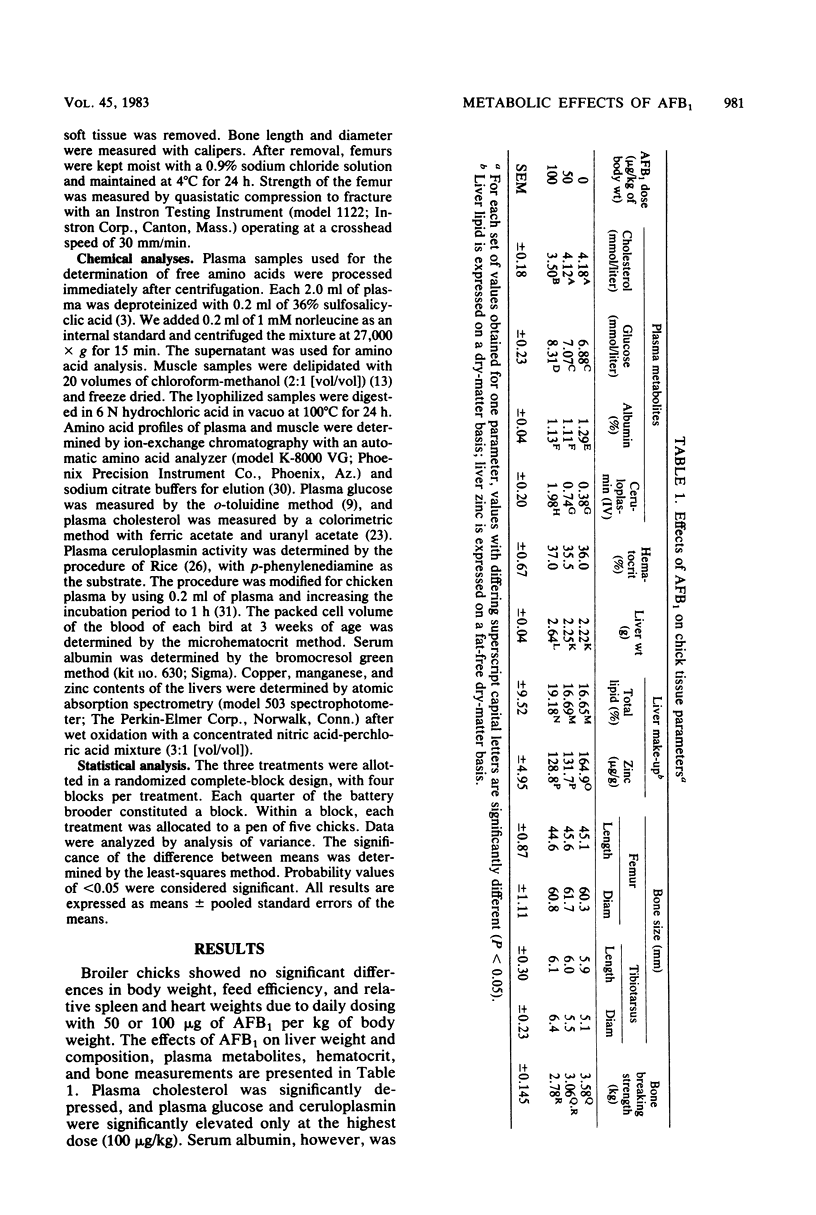
The effects of daily ingestion of aflatoxin B1 (AFB1) on growth, feed intake, plasma glucose, plasma cholesterol, plasma amino acids, plasma albumin, plasma ceruloplasmin, muscle amino acids, liver lipid, and bone strength were studied. For 3 weeks, beginning at an age of 2 days, broiler chicks were dosed daily per os with 50 or 100 micrograms of AFB1 per kg of body weight. Body weight and feed consumption were recorded daily, and metabolic responses were determined at 3 weeks. Treatment with AFB1 did not significantly alter body weight or feed intake. Relative liver weight showed a significant increase at the highest dose, with a significant concomitant increase in liver lipid and decrease in hepatic zinc. Relative spleen and heart weights were not affected by the toxin. Plasma glucose and cholesterol were significantly elevated at the highest dose. AFB1 significantly decreased plasma lysine and histidine and significantly increased muscle histidine, arginine, and valine. AFB1 decreased plasma albumin and markedly increased plasma ceruloplasmin. Dimensions of the long bones (femur and tibiotarsus) were not altered by the toxin. However, AFB1 caused a significant linear decline in the resistance of bone to breakage ("bone breaking strength"). The results indicate that low levels of AFB1 reduced bone strength in broiler chicks. The alterations in blood parameters indicated that AFB1 can disrupt metabolism even at low levels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird F. H. The effect of aflatoxin B1 on the utilization of cholecalciferol by chicks. Poult Sci. 1978 Sep;57(5):1293–1296. doi: 10.3382/ps.0571293. [DOI] [PubMed] [Google Scholar]
- Blankenship L. T., Dickey J. F., Bodine A. B. In vitro mycotoxin binding to bovine uterine steroid hormone receptors. Theriogenology. 1982 Mar;17(3):325–331. doi: 10.1016/0093-691x(82)90092-9. [DOI] [PubMed] [Google Scholar]
- Block W. D., Markovs M. E., Steele B. F. Comparison between free amino acid levels in plasma deproteinated with picric acid and with sulfosalicylic acid. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1089–1091. doi: 10.3181/00379727-122-31333. [DOI] [PubMed] [Google Scholar]
- Britton W. M., Wyatt R. D. Effect of dietary aflatoxin on vitamin D3 metabolism in chicks. Poult Sci. 1978 Jan;57(1):163–165. doi: 10.3382/ps.0570163. [DOI] [PubMed] [Google Scholar]
- Clifford J. I., Rees K. R. The interaction of aflatoxins with purines and purine nucleosides. Biochem J. 1967 May;103(2):467–471. doi: 10.1042/bj1030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J. Regulatory aspects of zinc metabolism in liver and intestine. Nutr Rev. 1979 Apr;37(4):97–103. doi: 10.1111/j.1753-4887.1979.tb02221.x. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- Donaldson W. E., Tung H. T., Hamilton P. B. Depression of fatty acid synthesis in chick liver (Gallus domesticus) by aflatoxin. Comp Biochem Physiol B. 1972 Apr 15;41(4):843–847. doi: 10.1016/0305-0491(72)90097-1. [DOI] [PubMed] [Google Scholar]
- FARE G., HOWELL J. S. THE EFFECT OF DIETARY COPPER ON RAT CARCINOGENESIS BY 3-METHOXY DYES. I. TUMORS INDUCED AT VARIOUS SITES BY FEEDING 3-METHOXY-4-AMINOAZOBENZENE AND ITS N-METHYL DERIVATIVE. Cancer Res. 1964 Aug;24:1279–1283. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fisher G. L., Shifrine M. Hypothesis for the mechanism of elevated serum copper in cancer patients. Oncology. 1978;35(1):22–25. doi: 10.1159/000225249. [DOI] [PubMed] [Google Scholar]
- Frieden E., Hsieh H. S. Ceruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol Relat Areas Mol Biol. 1976;44:187–236. doi: 10.1002/9780470122891.ch6. [DOI] [PubMed] [Google Scholar]
- Hamilton P. B. Interrelationships of mycotoxins with nutrition. Fed Proc. 1977 May;36(6):1899–1902. [PubMed] [Google Scholar]
- Huff W. E., Doerr J. A., Hamilton P. B., Hamann D. D., Peterson R. E., Ciegler A. Evaluation of bone strength during aflatoxicosis and ochratoxicosis. Appl Environ Microbiol. 1980 Jul;40(1):102–107. doi: 10.1128/aem.40.1.102-107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E. Liver disease and protein needs. Annu Rev Nutr. 1982;2:21–50. doi: 10.1146/annurev.nu.02.070182.000321. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Nakaue H., Deagen J. T., Whanger P. D., Arscott G. H. Accumulation and depletion of zinc in chick tissue metallothioneins. J Nutr. 1979 Oct;109(10):1720–1729. doi: 10.1093/jn/109.10.1720. [DOI] [PubMed] [Google Scholar]
- Patterson D. S. Metabolism as a factor in determining the toxic action of the aflatoxins in different animal species. Food Cosmet Toxicol. 1973 Apr;11(2):287–294. doi: 10.1016/s0015-6264(73)80496-1. [DOI] [PubMed] [Google Scholar]
- Raj H. G., Shankaran R., Venkitasubramanian T. A. Metabolic effects of aflatoxin in one day old chicks. Indian J Biochem. 1970 Mar;7(1):55–56. [PubMed] [Google Scholar]
- Rosen H. M., Yoshimura N., Hodgman J. M., Fischer J. E. Plasma amino acid patterns in hepatic encephalopathy of differing etiology. Gastroenterology. 1977 Mar;72(3):483–487. [PubMed] [Google Scholar]
- Rucker R. B., Riggins R. S., Laughlin R., Chan M. M., Chen M., Tom K. Effects of nutritional copper deficiency on the biomechanical properties of bone and arterial elastin metabolism in the chick. J Nutr. 1975 Aug;105(8):1062–1070. doi: 10.1093/jn/105.8.1062. [DOI] [PubMed] [Google Scholar]
- Shankaran R., Raj H. G., Venkitasubramanian T. A. Effect of aflatoxin on carbohydrate metabolism in chick liver. Enzymologia. 1970 Dec 30;39(6):371–378. [PubMed] [Google Scholar]
- Starcher B., Hill C. H. Hormonal induction of ceruloplasmin in chicken serum. Comp Biochem Physiol. 1965 Aug;15(4):429–434. doi: 10.1016/0010-406x(65)90143-x. [DOI] [PubMed] [Google Scholar]
- Tung H. T., Donaldson W. E., Hamilton P. B. Altered lipid transport during aflatoxicosis. Toxicol Appl Pharmacol. 1972 May;22(1):97–104. doi: 10.1016/0041-008x(72)90229-3. [DOI] [PubMed] [Google Scholar]
- Tung H. T., Wyatt R. D., Thaxton P., Hamilton P. B. Concentrations of serum proteins during aflatoxicosis. Toxicol Appl Pharmacol. 1975 Nov;34(2):320–326. doi: 10.1016/0041-008x(75)90038-1. [DOI] [PubMed] [Google Scholar]
- Voigt M. N., Wyatt R. D., Ayres J. C., Koehler P. E. Abnormal concentrations of B vitamins and amino acids in plasma, bile, and liver of chicks with aflatoxicosis. Appl Environ Microbiol. 1980 Nov;40(5):870–875. doi: 10.1128/aem.40.5.870-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


