Abstract
Natural killer T (NKT) cells are a T-cell subpopulation known to possess immunoregulatory functions and recognize CD1d molecules. The majority of NKT cells express an invariant T-cell receptor (TCR) α chain rearrangement (Vα14Jα18 in mice; Vα24Jα18 in humans) and are called type I NKT cells; all other NKT cells are type II. In the current study, we have analyzed the roles for these NKT-cell subsets in the host's innate antitumor response against a murine B-cell lymphoma model in vivo. In tumor-bearing mice, we found that type I NKT cells conferred protection in a CD1d-dependent manner, whereas type II NKT cells exhibited inhibitory activity. Pro- and anti-inflammatory cytokines secreted by splenocytes from tumor-bearing mice correlated with tumor progression. Myeloid cells (CD11b+Gr1+) were present in large numbers at the tumor site and in the spleen of tumor-bearing type I NKT–deficient mice, suggesting that antitumor immunosurveillance was inhibited by CD11b+Gr1+ cells. Overall, these data suggest that there are distinct roles for NKT-cell subsets in response to a B-cell lymphoma in vivo, pointing to potential novel targets to be exploited in immunotherapeutic approaches against blood cancers.
Introduction
Tumors must escape from the host's immune surveillance to survive and grow. Lymphoma is a general term for cancers that develop in the lymphatic system and it is the third most common cancer in children and adolescents.1 To improve the outcome in patients with hematopoietic tumors, a better understanding of the immunobiology of lymphoma is essential. A novel lymphocyte population that has been identified as a key player in both innate and acquired immune responses, including as antitumor effector cells, is the natural killer T (NKT) cell.2–4 NKT cells recognize lipid antigens presented by the MHC class I-like CD1d molecule and express cell surface markers shared with NK cells.3 The vast majority of these T cells are canonical or invariant NKT (type I NKT) cells that possess a specific T-cell receptor (TCR) α–chain rearrangement (Vα14Jα18 in mice; Vα24Jα18 in humans), associated with Vβ chains of limited diversity. All other NKT cells that are CD1d-restricted and do not express this invariant TCR are called type II NKT cells.5,6 Unlike type I NKT cells, little is known about type II NKT cells, but there is some evidence that type II NKT cells are a functionally important T-cell subset.5 As CD1d molecules are vital for NKT-cell development,7 mice lacking the CD1d1 gene (CD1KO mice) are deficient in all CD1d-restricted NKT cells (both type I and type II).8 Mice lacking the Jα18 gene (Jα18KO mice) are deficient only in type I NKT cells. As NKT cells are capable of secreting both Th1 and Th2 cytokines, this has made it difficult to predict the consequences of their activation in vivo but has nonetheless created much speculation that they play a central role in immunoregulation.2
NKT cells are directly cytotoxic and their activation can also result in “adjuvant effects” during antitumor immune responses by activating other cytotoxic lymphocytes, mainly through a Th1 cytokine cascade.4 However, there are reports demonstrating a suppressive antitumor role for CD4+ NKT cells in some murine tumor models,9 and type II NKT cells were shown to be capable of down-regulating tumor immunosurveillance.10 The role of NKT cells in the evasion of hematopoietic tumors from the host's innate antitumor immune response in vivo has only recently begun to be investigated, and we have reported that type I NKT cells play an inhibitory role in a murine T-cell lymphoma model.11 Several human hematopoietic cell types express CD1d on their surface,12 but the overall role of CD1d in antitumor immunity is not well understood. We have previously demonstrated that certain hematopoietic tumors shed glycolipids that mask CD1d-mediated antigen presentation to both type I and II NKT cells.13 Recently, in vitro killing of EL-4 T-cell lymphoblastic lymphoma cells by type I NKT cells and their in vivo eradication in a CD1d-dependent manner has been reported.14
CD11b and Gr-1 are the most common markers found on myeloid derived suppressor cells (MDSCs) and these cells are distinct from T lymphocytes and NK cells.15 Accumulating evidence since the 1980s has demonstrated that MDSCs of the myeloid macrophage/DC lineage, are significantly increased in the spleen and bone marrow of animals bearing large tumors and under conditions associated with impaired immune reactivity.16 The MDSC population is heterogenous, including mature granulocytes, immature macrophages, immature DCs, immature myeloid cells, monocytes, and a broad representation of immature cells of the myelomonocytic lineage.15 They express abnormally low levels of MHC class II molecules and low to undetectable levels of costimulatory molecules. They are unable to process and present antigens, and therefore do not induce effective antitumor cellular immune responses.16 Recently, Taniguchi and colleagues have reported that an NKT cell–mediated antitumor immune response could be enhanced by inhibiting MDSCs in B16 melanoma and 3LL Lewis lung cancer models.17
In the current report, we have studied the role(s) of both type I and type II NKT cells in the control of a B-cell lymphoma in vivo. We have found that type I NKT cells are protective, whereas type II NKT cells are suppressive in the antitumor immune response to this tumor.
Methods
Mice
Inbred BALB/c wildtype mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CD1d1-deficient (CD1KO) mice8 were obtained from L. Van Kaer (Vanderbilt University, Nashville, TN). Jα18KO mice18 were kindly provided by Dr R. Singh (University of Cincinnati, OH) with permission from Dr M. Taniguchi (Chiba University, Chiba, Japan). All gene-targeted mice were backcrossed onto the BALB/c background for 10 generations. All mice were age- and sex-matched and used between 6 and 12 weeks of age. All animal procedures were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Antibodies
The mouse CD1d-specific 1H6 mAb has been previously described.19 1H6 ascites and the isotype control mAb HB158 hybridoma supernatant were purified on a protein A column for in vivo use. Phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated antibodies specific for mouse CD4, CD8, TCRβ, B220, Gr-1, CD11b, CD44, and DX5 were purchased from BD-PharMingen (San Diego, CA). PE-conjugated rabbit anti–mouse Ig antiserum was purchased from Dako (Carpinteria, CA). Purified and biotinylated antibodies specific for mouse transforming growth factorβ (TGFβ) and IL-13 were purchased from R&D Systems (Minneapolis, MN), whereas those specific for mouse IL-2, IL-12, IFN-γ, and GM-CSF were purchased from BD-PharMingen. All the cytokine standards used in the enzyme-linked immunosorbent assays (ELISA) were purchased from PeproTech (Rocky Hill, NJ).
Tumor cell lines and murine type I NKT cells
The B-cell lymphoma cell line NS0 was kindly provided by S. Joyce (Vanderbilt University). NS0 cells were transfected with the pSRα-neo vector alone (NS0-V) or the vector with a cd1d1 cDNA insert (NS0-CD1) by standard electroporation techniques. Isolation of murine type I NKT cells was performed as previously described.20 Briefly, isolated liver mononuclear cells (LMNC),21 were blocked with anti-FcRγII (2.4G2, ATCC, Manassas, VA) and treated with recombinant soluble dimeric mouse CD1d-Ig fusion proteins (DimerXI; BD Biosciences) loaded with a 40 M excess of α-galactosylceramide (α-GalCer) and labeled with a Zenon R-PE mouse IgG1 (Invitrogen, Carlsbad, CA) as described previously.20 In some experiments, PBS-57-loaded allophycocyanin-conjugated CD1d tetramers (National Institutes of Health [NIH] National Institute of Allergy and Infectious Diseases [NIAID] Tetramer Facility at the Emory University Vaccine Center, Atlanta, GA) were used. Unloaded CD1d1 dimers conjugated with Zenon R-PE mouse IgG1 and unloaded allophycocyanin-conjugated CD1d tetramers were used as negative controls. Dimer or tetramer-positive cells were electronically sorted using a BD FACSAria cell sorter.
In vivo tumor assays
Single cell suspensions of tumor cells were prepared in Iscove modified Eagle medium (IMDM) containing 5% fetal bovine serum (FBS) and injected intraperitoneally. Liver-derived NKT cells from BALB/c mice were adoptively transferred on the indicated day's posttumor inoculation to Jα18KO mice, intravenously. To block CD1d in vivo, 50 μg purified 1H6 or isotype control mAb in PBS was injected on the indicated day's posttumor inoculation to BALB/c mice, intraperitoneally.
Ex vivo cytokine analyses, flow cytometry, and NKT-cell functional assays
Ex vivo cytokine analysis from splenocytes of tumor-bearing mice was performed as previously described.11 Briefly, single cell suspensions of splenocytes from NS0-V- or NS0-CD1-inoculated BALB/c wildtype, CD1KO, and Jα18KO mice were prepared from tumor-bearing mice on day 25 and 50 after tumor inoculation. The cells were cultured at a density of 3 × 106 cells/well in a 24-well plate along with irradiated (5000 rads) NS0-V- or NS0-CD1 cells (7.5 × 105 tumor cells/well) at a 4:1 ratio. Two days after the coculture, supernatants were harvested and stored at −20°C until used for cytokine measurement by ELISA. All samples were analyzed in triplicate. Supernatants from NS0-V- or NS0-CD1 cells, and splenocytes of tumor and non–tumor-bearing mice cultured alone were included as controls in the ELISA assays. The background cytokine levels were subtracted from the experimental values. Single cell suspensions of splenocytes harvested from NS0-V– or NS0-CD1–bearing mice for FACS analyses of the MDSC population was performed as previously described.11 The murine NKT-cell hybridoma assay was performed as described.19 Briefly, NS0-V- or NS0-CD1 cells were cocultured with murine NKT-cell hybridoma DN32.D322,23 at 1:1 ratio for 48h, supernatants harvested and IL-2 production was measured by ELISA as previously described.19 The cytotoxic activity of murine NKT cells against NS0-V or NS0-CD1 cells was performed as previously described.24
Statistical analysis
For the statistical analysis of tumor growth in mice, the log-rank test was performed using GraphPad PRISM software (version 4.00 for Windows; GraphPad, San Diego, CA). A nonparametric statistical analysis using SAS, version 9 (SAS Institute, Cary, NC) was performed for comparing the levels of cytokines secreted by different tumor-bearing mouse strains. Two experiments (n = 6) were combined (P > .05, Wilcoxon test) for the statistical analysis of those data. An overall test of difference between strains was performed using the Kruskal-Wallis test. Pairwise comparisons between strains were performed using Wilcoxon tests with a Bonferroni adjustment for multiple comparisons.
Results
Type I NKT cells are essential for the survival of mice against a B-cell lymphoma
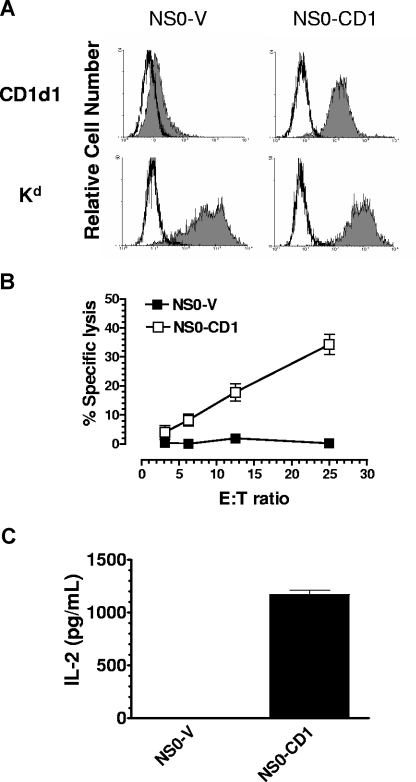
CD1d molecules are expressed on B cells, macrophages, dendritic cells, thymocytes and some hematopoietic tumor cells.12,14,25 The role of CD1d on tumor cells in the host's antitumor immune response has not been well established. The B-cell lymphoma NS0 naturally expresses a very low level of CD1d on the cell surface (Figure 1A). Upon expression of mouse CD1d1 in NS0 cells (NS0-CD1), these cells were able to stimulate cytokine production by NKT cells and were susceptible to NKT cell–mediated lysis in vitro (Figure 1B,C). Vector control NS0 (NS0-V) cells failed to activate NKT cells and were not killed by NKT cells in vitro. These results are consistent with a previous report showing that the sensitivity of leukemia cells to NKT cell–mediated lysis is correlated with CD1d expression.12 Some glycolipid fractions from the tumor cell membrane are known to be presented by CD1d and to be recognized by NKT cells,4 although we have reported in at least one case that tumor-derived glycolipids can be inhibitory.13 Our in vitro experiments using NS0 cells suggested that tumor-derived lipid antigens are capable of being presented by CD1d molecules and activating the lytic effector program of NKT cells.
Figure 1.
The parental murine B-cell lymphoma NS0 is deficient for cell surface CD1d expression and does not stimulate NKT cells. (A) NS0-V and NS0-CD1 cells were stained for mouse CD1d (or Kd) and analyzed by flow cytometry. Open histogram: isotype control; filled histogram: anti-mouse CD1d or Kd. (B) NKT cells kill only CD1d-expressing NS0 cells. Splenic NKT cells were used as effectors against 51Cr-labeled NS0-V (■) or NS0-CD1 (□) target cells in a 24-hour 51Cr release assay. (C) NKT cells are activated only by CD1d-expressing NS0 cells. NS0-V or NS0-CD1 cells were cocultured with the NKT-cell hybridoma DN32.D3 for 48 hours. The supernatant was tested for IL-2 production as an indicator of NKT-cell activation. Each bar represents triplicate determinations, and the data are shown as mean plus SD. The data shown are representative of 3 independent experiments.
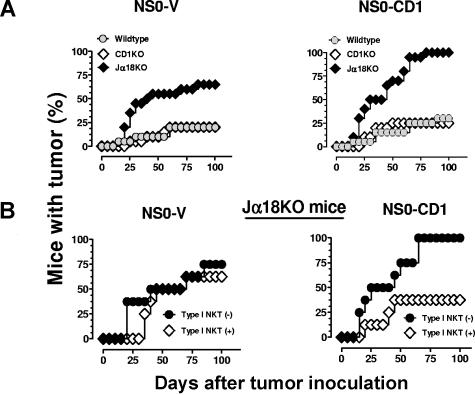
The in vivo role for NKT cells in the control of B-cell lymphomas and the importance of CD1d in the antitumor immune response against these tumors are unknown. To address these questions, syngenic BALB/c wildtype, CD1KO, and Jα18KO mice were inoculated intraperitoneally with NS0-V or NS0-CD1 cells. Ascites tumor development was detected as early as day 25 after tumor inoculation and 5 to 10 days later, the mice exhibited severe morbidity and rapid mortality. More than 70% of the Jα18KO mice inoculated with NS0-V tumors died within 100 days, with a mean survival time (MST) of 61 days, whereas all of the NS0-CD1–bearing Jα18KO mice died within 100 days (MST = 45 days; Figure 2A). Less than 30% of the wildtype and CD1KO mice succumbed to either tumor; these mice suffered less and survived longer compared with Jα18KO mice (Figure 2A and Table 1). The percentage of Jα18KO mice bearing NS0-CD1 tumors was higher than those inoculated with the control tumor (P = .0153) and this may have been due to tumor cell CD1d-mediated activation of type II NKT cells in the absence of protective type I NKT cells. Thus, these results suggested that type I NKT cells were protective and type II NKT cells were suppressive in this tumor model. This observation was confirmed by the enhanced survival after adoptive transfer of electronically sorted liver type I NKT cells from wildtype BALB/c mice to tumor-bearing Jα18KO mice (P = .0163; Figure 2B). Notably, this protection depended upon CD1d being expressed by the tumor. Therefore, these results confirmed that type I NKT cells are responsible for protecting mice from rapid death by a B-cell lymphoma, and this protective effect is CD1d-dependent. Because both type I and type II NKT cells have opposite functions,2,3,5 the similar growth of NS0-V and NS0-CD1 tumors in wildtype or CD1d-deficient mice (the latter of which has neither type I nor type II NKT cells) was predicted. Furthermore, the adoptive transfer of type I NKT cells reduced the tumor incidence in NS0-CD1–bearing Jα18KO mice to the levels observed in wildtype mice (Figure 2B right panel), also consistent with our hypothesis. However, why was there no change in the tumor incidence in Jα18KO mice bearing NS0-V control tumors that were adoptively-transferred with NKT cells (Figure 2A left panel)? It is known that upon activation, NKT cells can stimulate NK cells,26,27 and these 2 lymphocyte populations can work together or independently in various tumor models.28 With hematopoietic tumors in particular, there is an inverse correlation between MHC class I expression on the cells and their susceptibility to NK cell–mediated lysis.29 However, with the very high level of surface MHC class I (eg, H2-Kd) on the NS0-V cells (Figure 1A), NK activation likely would effectively be irrelevant. Hence, no difference in NS0-V growth in the presence or absence of type I NKT cells in Jα18KO mice was observed (Figure 2B left panel). Thus, overall, the data strongly suggest that type I NKT cells serve as antitumor effector cells, whereas type II NKT cells act opposite to their type I counterparts.
Figure 2.
Protective antitumor immune response mediated by type I NKT cells to a murine B-cell lymphoma. (A) BALB/c wildtype ( ), CD1KO (◇), and Jα18KO (♦) mice were inoculated with 107 NS0-V or NS0-CD1 cells. The results consist of the pooled data from 3 independent experiments (n = 20). The log-rank test was used to determine P values. Tumor incidence in mice inoculated with NS0-V cells compared with NS0-CD1 cells: wildtype, P = .537; in CD1KO, P = .6560; and in Jα18KO mice, P = .015. (B) BALB/c Jα18KO mice were inoculated with tumors as above and on days 0, 10, and 20, the mice received 2.5 × 105 electronically-sorted type I NKT cells by adoptive transfer or sham, intravenously. The results shown are pooled data (4 mice/experiment; n = 8). The log-rank test was used to determine P values for tumor-bearing mice receiving sham treatment (●) or NKT cells (◇) and NS0-V (P = .6130) or NS0-CD1 cells (P = .0163).
), CD1KO (◇), and Jα18KO (♦) mice were inoculated with 107 NS0-V or NS0-CD1 cells. The results consist of the pooled data from 3 independent experiments (n = 20). The log-rank test was used to determine P values. Tumor incidence in mice inoculated with NS0-V cells compared with NS0-CD1 cells: wildtype, P = .537; in CD1KO, P = .6560; and in Jα18KO mice, P = .015. (B) BALB/c Jα18KO mice were inoculated with tumors as above and on days 0, 10, and 20, the mice received 2.5 × 105 electronically-sorted type I NKT cells by adoptive transfer or sham, intravenously. The results shown are pooled data (4 mice/experiment; n = 8). The log-rank test was used to determine P values for tumor-bearing mice receiving sham treatment (●) or NKT cells (◇) and NS0-V (P = .6130) or NS0-CD1 cells (P = .0163).
Table 1.
Statistical analysis of tumor growth in wildtype, CD1KO, and Jα18KO mice inoculated with NS0-V or NS0-CD1 cells (n = 20 mice/group)*
| Tumor and mouse group† | Mice with tumors |
|
|---|---|---|
| P | Significance‡ | |
| NS0-V with wildtype and CD1KO | .993 | NS§ |
| NS0-V with wildtype and Jα18KO | .003 | S¶ |
| NS0-V with CD1KO and Jα18 KO | .003 | S |
| NS0-CD1 with wildtype and CD1KO | .792 | NS |
| NS0-CD1 with wildtype and Jα18 KO | <.001 | S |
| NS0-CD1 with CD1KO and Jα18KO | <.001 | S |
| Wildtype with NS0-V and NS0-CD1 | .537 | NS |
| CD1KO with NS0-V and NS0-CD1 | .656 | NS |
| Jα18KO with NS0-V and NS0-CD1 | .015 | S |
Mice were inoculated with 107 tumor cells/mouse, intraperitoneally.
Comparisons were made between the different combinations of groups in order to determine the statistical significance of the analyses.
The P values are based on the log-rank test comparing either two groups of mice with one cell line or two cell lines with one group of mice as indicated.
Not significant.
Significant (entries in bold).
Liver NKT cells are considered to be functional representatives of all invariant NKT cells in mice.30 In an earlier study, it was found that 2.5 × 105 liver NKT cells injected once to tumor-bearing C57BL/6 Jα18KO mice were sufficient to confer substantial protection of mice from tumor burden.30 Thus, additionally, our data suggest that a similar number of BALB/c liver NKT cells injected 3 times is also significantly protective in a B-cell lymphoma (and CD1d+) model.
Enhanced mortality of tumor-bearing Jα18-deficient mice is due to type II NKT cells
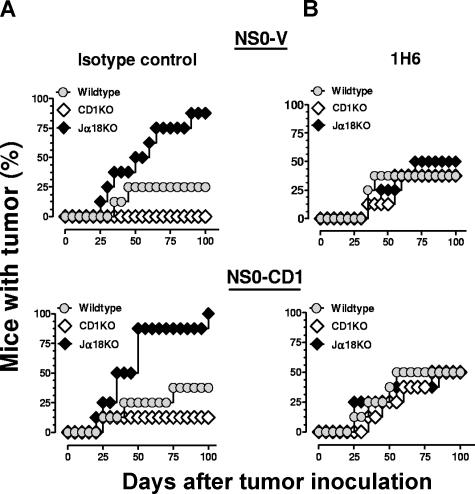
The function of type II NKT cells in vivo can be assessed by comparing results from experiments using Jα18- and CD1d-deficient mice.4,10 Jα18KO mice only lack type I NKT cells, whereas CD1KO mice are deficient in both type I and type II. Therefore, considering the reduced survival in Jα18KO compared with CD1KO mice (Figure 2A), we speculated that type II NKT cells were playing an immunosuppressive role. The recruitment and activation of type I NKT cells is an important consideration during the immunotherapy of early stage tumors.31 In our B-cell lymphoma model, a similar recruitment and activation of type II NKT cells appeared to be responsible for inhibiting the host's innate antitumor immunosurveillance. To address this question, tumor-bearing mice were injected with a mouse CD1d-specific mAb (1H6) that blocks CD1d-dependent NKT-cell activation in vitro19 or an isotype control mAb. It was found that the 1H6 mAb completely restored the level of survival in tumor-bearing Jα18KO mice to that observed in wildtype mice (Figure 3; Table 2). Furthermore, 1H6 can substantially impair cytokine production by NKT cells after injection of α-GalCer in vivo (data not shown). These data are supported by an earlier study showing that the in vivo function of NKT cells could be blocked using an anti-CD1d antibody.10 These results further confirmed a possible suppressive function for type II NKT cells against a B-cell lymphoma. The antibody-mediated CD1d block in wildtype mice also did not alter tumor incidence, suggesting that inhibiting both NKT-cell subsets has no effect on survival in this tumor model.
Figure 3.
Inhibition of the antitumor immune response by type II NKT cells in tumor-bearing mice. NS0-V or NS0-CD1 cells (107) were injected intraperitoneally into BALB/c wildtype ( ), CD1KO (◇) and Jα18KO (♦) mice. On days −1, 5, 10, 20, 30, 40, and 50, the mice were injected intraperitoneally with 50 μg of isotype control antibody (A) or the anti-mouse CD1d antibody 1H6 (B). Tumor growth was monitored for 100 days. The results shown are pooled data from 2 independent experiments (4 mice/experiment; n = 8).
), CD1KO (◇) and Jα18KO (♦) mice. On days −1, 5, 10, 20, 30, 40, and 50, the mice were injected intraperitoneally with 50 μg of isotype control antibody (A) or the anti-mouse CD1d antibody 1H6 (B). Tumor growth was monitored for 100 days. The results shown are pooled data from 2 independent experiments (4 mice/experiment; n = 8).
Table 2.
Statistical analysis of tumor growth in BALB/c wildtype, CD1KO, and Jα18KO mice inoculated with NS0-V or NS0-CD1 cells and treated with an isotype control or anti-CD1d mAb (n = 8 mice/group)*
| Tumor and mouse group† | In vivo CD1d block |
|||
|---|---|---|---|---|
| Isotype control |
Anti-mouse CD1d |
|||
| P | Significance‡ | P | Significance | |
| NS0-V with wildtype and CD1KO | .144 | NS§ | .843 | NS |
| NS0-V with wildtype and Jα18KO | .021 | S¶ | .779 | NS |
| NS0-V with CD1KO and Jα18 KO | <.001 | S | .612 | NS |
| NS0-CD1 with wildtype and CD1KO | .283 | NS | .846 | NS |
| NS0-CD1 with wildtype and Jα18 KO | .040 | S | .926 | NS |
| NS0-CD1 with CD1KO and Jα18KO | .006 | S | .943 | NS |
| Wildtype with NS0-V and NS0-CD1 | .609 | NS | .665 | NS |
| CD1KO with NS0-V and NS0-CD1 | .317 | NS | .690 | NS |
| Jα18KO with NS0-V and NS0-CD1 | .364 | NS | .943 | NS |
Mice were inoculated with 107 tumor cells/mouse and 50 μg of isotype or anti-mouse CD1d mAb on days −1, 5, 10, 20, 30, 40, and 50 intraperitoneally.
Comparisons were made between the different combinations of groups in order to determine the statistical significance of the analyses.
The P values are based on the log-rank test comparing either 2 groups of mice with 1 cell line or 2 cell lines with 1 group of mice as indicated.
Not significant.
Significant (entries in bold).
Pro- and anti-inflammatory cytokine profiles reflect tumor progression
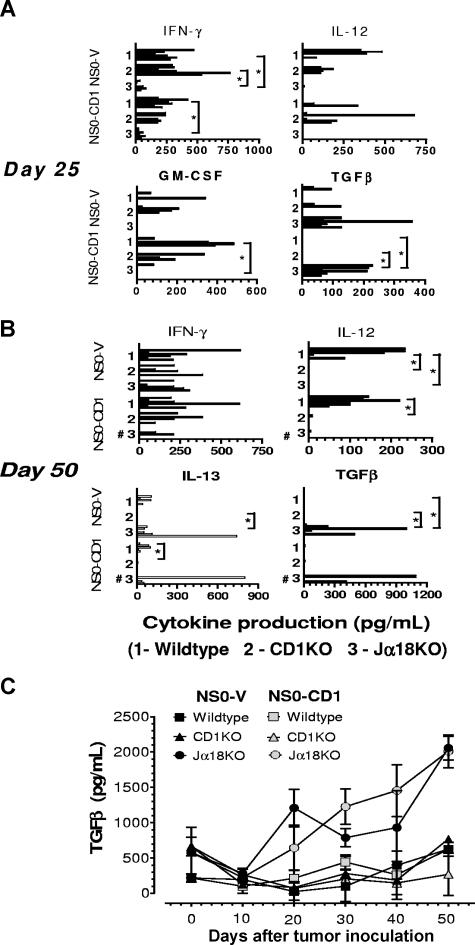
In other murine tumor model systems, it has been reported that CD4+ NKT cells exert an inhibitory effect on antitumor immunosurveillance mediated through the actions of anti-inflammatory cytokines like IL-13.9 Similarly, we have reported in a murine T-cell lymphoma model that Jα18KO and CD1KO mice produce lower levels of Th2 cytokines and survive longer than wildtype mice.11 In the current study, to explore how type I and II NKT-cell subsets mediated the divergent antitumor immune response to a B-cell lymphoma, the pro- and anti-inflammatory cytokine profiles in tumor-bearing wildtype and mutant mice at various stages of tumor growth were assessed. Our in vivo experiments indicated that the maximum ascites tumor development was detectable between days 25 and 60 after inoculation. Therefore, spleens from wildtype, CD1KO, and Jα18KO mice bearing NS0-V and NS0-CD1 tumors were harvested on day 25 or 50, splenocytes were cocultured with the respective irradiated tumor cells, and the production of IFN-γ, GM-CSF, IL-12, TGFβ, and IL-13 into the supernatant was measured (Figure 4). Secretion of the pro-inflammatory cytokines IFN-γ and IL-12 were significantly higher in wildtype and CD1KO mice compared with Jα18KO mice (P < .05; Figure 4A,B). In contrast, the levels of the anti-inflammatory cytokines TGFβ and IL-13 were significantly higher in Jα18KO mice and nearly undetectable in wildtype and CD1KO mice (Figure 4A,B). TGFβ is a cytokine that has been shown to block cytotoxic T lymphocyte–mediated tumor immunosurveillance.32 We detected high levels of serum TGFβ in Jα18KO mice at various stages of tumor growth compared with wildtype and CD1KO mice (Figure 4C). We did not detect any cytokine by intracellular staining and FACS analysis in splenocytes from tumor-bearing mice (data not shown).
Figure 4.
Levels of pro- and anti-inflammatory cytokines are indicators of murine B cell lymphoma growth in vivo. Splenocytes from BALB/c wildtype, CD1KO, and Jα18KO mice inoculated with NS0-V or NS0-CD1 cells were collected 25 (A) or 50 (B) days later and cocultured with irradiated tumor cells. The culture supernatants were tested for the indicated cytokines by ELISA. Each bar represents the cytokine production from individual mice (n = 6 from 2 independent experiments). No bar in a group indicates that the cytokine was below the level of detection. In the day 50 NS0-CD1 tumor-bearing Jα18KO mouse group; 3 mice were dead by day 50 (#) and hence, cytokine production could not be measured. (C) BALB/c mice were inoculated with the tumors as above, and on days 0, 5, 10, 20, 30, 40, and 50, mice were bled and the serum assayed for TGFβ by ELISA. Error bars represent SD. In panels A and B, the asterisk indicates a significant P value (< .025) as determined by the Kruskal-Wallis test for the comparisons made between the indicated groups.
IL-12 has been shown to possess potent antitumor activity in a wide variety of murine tumor models via NK and/or NKT cells.33 As NKT cells are activated by the IL-12 produced during a tumor-induced inflammatory process, NKT cells might be able to respond to any type of tumor.4 We also detected significantly higher levels of IL-12 in wildtype mice (Figure 4A,B), correlating with a reduced tumor incidence and is supportive of a possible protective function mediated by type I NKT cells.
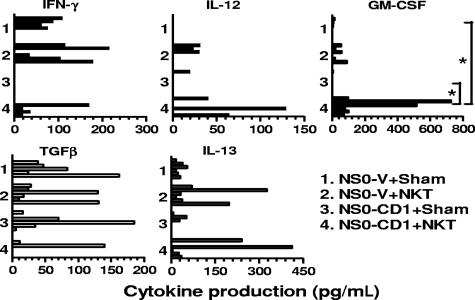
Cytokine production from splenocytes of tumor-bearing Jα18KO mice adoptively transferred with type I NKT cells was analyzed as well. The results obtained support the survival data (Figure 2), whereby the pro-inflammatory cytokines IFN-γ, IL-12, and GM-CSF were detected more in Jα18KO mice bearing NS0-CD1 and adoptively transferred with NKT cells as opposed to those that did not (Figure 5). A similar but reduced trend in cytokine production was detected in NS0-V–bearing Jα18KO mice receiving NKT cells (Figure 5). The anti-inflammatory cytokine TGFβ was detected in more sham-treated NS0-CD1–bearing mice than in those receiving NKT cells. It is important to note that the reduced tumor incidence in NS0-CD1–bearing Jα18KO mice after NKT cell transfer was not complete, although it was significant. This suggests that the adoptively-transferred type I NKT cells induced a prevalence of pro-inflammatory cytokine production in tumor-bearing mice. FACS analyses of splenocytes from NS0-CD1–bearing Jα18KO mice receiving NKT cells showed a marginal rescue in CD4+ and CD8+ T-cell numbers and with a concomitant reduction in CD11b+Gr1+ cells compared with the control group (data not shown).
Figure 5.
Pro- and anti-inflammatory cytokine production correlates with survival of tumor-bearing Jα18KO mice adoptively transferred with type I NKT cells. BALB/c Jα18KO mice were inoculated with 107 NS0-V or NS0-CD1 tumors and on days 0, 10, and 20 received 2.5 × 105 electronically sorted type I NKT cells or sham intravenously. Splenocytes were collected 25 days later and cocultured with irradiated tumor cells for 2 days. The culture supernatants were tested for the indicated cytokines by ELISA. Each bar represents the cytokine production from individual mice (n = 6 from 2 independent experiments). No bar indicates that the cytokine was not detectable. The asterisk indicates a significant P value (< .025) as determined by the Kruskal-Wallis test for the comparisons made between the indicated groups.
Tumor-bearing type I NKT cell–deficient mice have elevated levels of myeloid suppressor cells
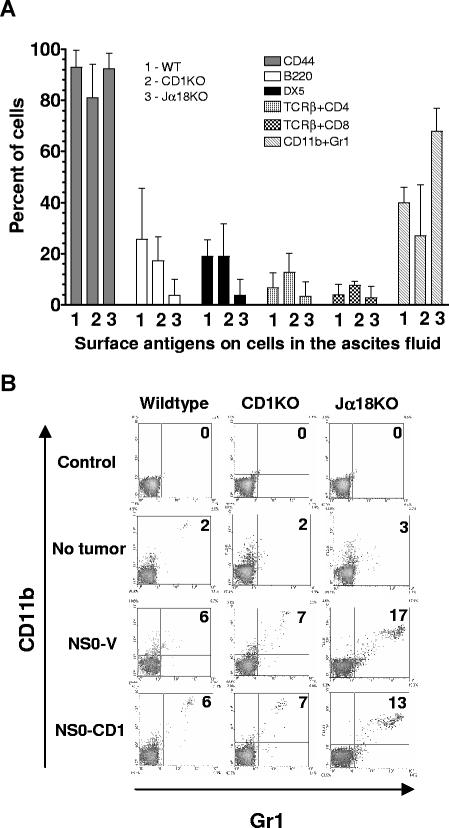
Necropsy analyses of tumor-bearing mice revealed metastases in the liver, spleen (3-10 times larger than normal), kidney, lymph nodes, intestines, and peritoneum. The host's response to prevent tumor progression is reflected by the predominant tumor-infiltrating leukocytes and other cells, which participate in the neoplastic process by fostering tumor cell proliferation, survival, and metastasis.34 To analyze the tumor microenvironment in the current study, ascites tumor cells from wildtype, CD1KO, and Jα18KO mice inoculated with NS0-V or NS0-CD1 cells were stained with various mAb to permit the identification of the individual leukocyte populations present. Interestingly, most of the ascites tumors had 2 or 3 distinct cell populations and tumor cells from each mouse had various levels of B220, DX5, TCRβ+CD4+, TCRβ+CD8+, and CD11b+Gr1+ cells (Figure 6A). Interestingly, we found lower numbers of DX5+ and B220+ cells, but substantially higher numbers of myeloid suppressor cells (MDSC; CD11b+Gr1+) in the ascites from tumor-bearing Jα18- (type I NKT cell)–deficient mice (Figure 6A). Furthermore, elevated levels of MDSC were detected in the spleens of these mice (Figure 6B).
Figure 6.
Ascites from tumor-bearing Type I NKT cell–deficient mice have elevated levels of myeloid suppressor (CD11b+Gr1+) cells. (A) NS0-V or NS0-CD1 tumor-bearing BALB/c wildtype, CD1KO, and Jα18KO mice were euthanized, and ascites tumor cells were harvested and stained for surface expression of CD44, B220, DX5, TCRβ and CD4, TCRβ and CD8, or CD11b and Gr1 and analyzed by flow cytometry. Each bar corresponds to the mean percentage of positive cells for a specific antigen obtained from 3 individual mice in each group (+ SD). (B) Splenocytes harvested from the above groups of mice were stained for Gr1 and CD11b. A representative FACS analysis of 3 individual mice is shown. Numbers on the plots indicate the percentage of total cells in that quadrant.
Discussion
Unlike murine NKT cells, the number of type I NKT cells in humans is highly variable among individuals and they represent only a small portion of total NKT cells, whereas type II NKT cells are the major population.35–40 Therefore, it is very essential to delineate the independent roles of NKT cell subsets in antitumor immunity to design effective immunotherapeutic strategies. Our previous studies have identified a suppressive antitumor immune response mediated by type I NKT cells in a murine T-cell lymphoma model.11 Interestingly, our in vivo results in the current study demonstrate opposing roles for type I and type II NKT cells. Differences in the immune response between Jα18KO and CD1KO mice have been observed in a protozoan disease model (Trypanosoma cruzi), with Jα18KO mice more susceptible to disease.41 Recently, opposite immunoregulatory functions by type I and type II NKT cells during the acute phase of schistosomiaisis has been reported.42 Enhanced tumor incidence in wildtype and Jα18KO compared with CD1KO mice has been observed by Berzofsky and colleagues in models of fibrosarcoma (15-12RM), colon carcinoma (CT26), and mammary carcinoma (4T1), suggesting a suppressive role for type II NKT cells, but a role for type I NKT cells in wildtype mice in those models was not demonstrated.10
We attempted to address the role of both CD1d molecules and NKT cells in a single tumor model system. Although B cells normally express CD1d on their surface, we found that the NS0 B-cell lymphoma has low/undetectable levels of CD1d. Thus, we hypothesized that down-regulation of CD1d is one means by which a B-cell lymphoma could evade the host's innate antitumor immune response. Because of this, we overexpressed CD1d in NS0 cells and compared it to the same tumor without CD1d overexpression. The role of CD1d expressed on NS0 tumors was evident in tumor-bearing Jα18KO mice, as NS0-CD1 tumors killed more mice with a shorter mean survival time than those inoculated with NS0-V tumors. The adoptive transfer of type I NKT cells significantly protected only NS0-CD1–bearing Jα18KO mice. The absence of CD1d on NS0-V cells is the likely reason why adoptively transferred type I NKT cells failed to protect NS0-V-bearing Jα18KO mice; the cytokine profile data correlated with this observation. However, it cannot be ruled out that the adoptive transfer of more type I NKT cells would reduce the tumor incidence in NS0-V–bearing Jα18KO mice. That being said, other lymphocyte populations do have the capacity to participate in antitumor immune responses. In particular, NK cells are well-known antitumor effector cells and are highly lytic against hematopoietic tumors both in vivo and in vitro.29,43,44 The function of NK cells can be affected by activated NKT cells.26,27 Furthermore, it is known that NK cells can work with (or can function independently of) NKT cells in a variety of tumor cell models.28,45,46 Thus, one might have predicted that the adoptive transfer of type I NKT cells could have had an effect on NS0-V tumor growth in Jα18KO mice through the action of NK cells. As indicated above, no difference in NS0-V tumor incidence was observed in Jα18KO mice regardless of whether type I NKT cells were adoptively transferred or not. Considering the high levels of MHC class I molecules on NS0 cells (Figure 1A), and the well-known inverse correlation between MHC class I levels and susceptibility to lysis by NK cells,29 this result may not be that surprising.
Recently, it has been demonstrated that interactions between type II NKT cells and hepatic DCs result in the regulation of type I NKT-cell activity in concanavalin A–induced hepatitis47; it is important to note that BALB/c mice are mainly Th2 cytokine-biased.48 Some human tumor cells express CD1d and are able to present glycolipids to NKT cells, eliciting IFN-γ release and cytolysis.49,50 GD3, a ganglioside expressed on the CD1d-negative human melanoma cell line SK-MEL-28, can be cross-presented by murine APC in a CD1d-dependent manner in vivo and this is a putative mechanism for NKT recognition of tumor glycolipids in CD1d− tumors.51 Therefore, cross-presentation of tumor-derived glycolipids by professional antigen presenting cells such as dendritic cells to NKT cells can be very important for the development of antitumor immunity.51,52 Were cross-presentation to be the major mechanism operative in our NS0 system, we would expect there to be a comparable reduction in NS0-V and NS0-CD1 tumor growth in Jα18KO mice receiving type I NKT cells (dendritic cells in these animals are CD1d+). As there was only a decrease in NS0-CD1 tumor growth upon the adoptive transfer of type I NKT cells in Jα18KO mice, it is likely that cross-presentation plays a more important role when the tumors are CD1d−.
We demonstrated the suppressive role for type II NKT cells to a B-cell lymphoma NS0 as indicated by increased tumor incidence in Jα18KO mice bearing both NS0-V or NS0-CD1 tumors and which is supported by significantly reduced tumor incidence in CD1KO mice. These results correlate with recent findings using CD1d− tumors as well10 and therefore potentially suggest an even more significant immunoregulatory (albeit negative) role played by type II NKT cells in the host's innate antitumor immune response. The absence of type II NKT cells in CD1KO mice would therefore not prohibit other immune cells (eg, NK cells and tumor-specific cytotoxic T lymphocytes [CTL]) from clearing the tumor burden and resulting in a significantly reduced tumor incidence. Thus, our work has revealed an important protective antitumor effect mediated by type I NKT cells against a B-cell lymphoma. This is the first unique (and CD1d+) tumor model study demonstrating distinct, independent and contrasting antitumor functions for the individual NKT cell subsets, providing useful information that can potentially be applied to NKT cell–based antitumor immunotherapy.
The progressive lethal tumor growth in 30% of the wildtype mice bearing either NS0-V or NS0-CD1 observed in the current study is similar to that found with the BW-Sp3 lymphoma in syngenic AKR mice, where a marked increase in MDSC was detected in those mice as well.15 The local effects of inhibitory cytokines in both lymphoid organs and at the tumor site by myeloid cells can thus target the generation of antitumor CTL with tumor progression occurring as a result.15 Studies using the BW-Sp3 lymphoma line, MZ1851RC and MZ1851LN renal carcinomas, and B16 melanoma have shown that Th2 cytokines dominate the host's response during the progressive growth stage of the tumor.15 Our in vivo analyses in a B-cell lymphoma model demonstrate how the effectiveness of NKT cell–dependent antitumor immunosurveillance can be predicted based on the cytokines produced.
It is known that mice bearing various types of tumors (either by inoculation or carcinogen-induced) progressively accumulate MDSC in the spleen and blood, and they are profoundly impaired in their immune response to various tumor-associated antigens.15 It has been reported that MDSC from Jα18KO mice in a pancreatic islet transplant model failed to produce IFN-γ; the production of IFN-γ in that model was dependent on the activation status of type I NKT cells.53 IL-13 produced from NKT cells activates CD11b+Gr1+ cells to produce TGFβ which directly suppresses tumor-specific CD8+ CTLs.32 The results from our study suggest that the CD11b+Gr1+ cells found in significant numbers in the ascites of tumor-bearing Jα18KO mice probably produced higher levels of TGFβ, (rather than IFN-γ) which resulted in the impairment of antitumor immunosurveillance.
We have detected very high expression of CD44 in all the ascites tumor cells (> 90%); this molecule is often used as a marker of a tumor's metastatic potential.54 Hematopoiesis in a tumor-bearing host is generally altered, manifested by an increase in CD11b+Gr1+ cells in both spleen and ascites; these cells directly promote neoplastic growth by decreasing tumor cell apoptosis and necrosis.16 This correlates very well with our observations in the current study, where we have observed a similar increase in the MDSC population (especially in type I NKT cell–deficient mice) by 4- to 6-fold in the spleen and in the ascites tumors. Thus, our findings suggest that there is active immunosurveillance at the tumor site. Depending on the final outcome, there is a shift in the immune cell populations that will either favor survival or death in the tumor-bearing host. Furthermore, the effects of the NKT-cell subsets in vivo may be regulated by the levels of Th1 and Th2 cytokines, and ultimately through MDSCs. Conventional treatments of hematologic malignancies using chemotherapeutic agents are often unsuccessful due to severe side effects and incomplete long term remission. Therefore, the availability of a novel immune-based therapeutic option would be of great interest. B-cell malignancies are considered to be the most responsive types of all human cancers in an immunotherapeutic setting.55 Our study has clearly demonstrated the importance of NKT-cell subsets in the host's innate antitumor immunity against a B-cell lymphoma. This knowledge will be beneficial in the design of NKT cell–based immunotherapeutic strategies against these tumors.
Acknowledgments
We thank Drs L. Van Kaer, R. Singh, and M. Taniguchi for mice. Dr V. Sriram provided help during type I NKT-cell isolation. K. Gillett-Heacock, C. Willard, J. Eltz, and B. Champ provided expert technical assistance. Dr H. Twigg and P. Smith provided invaluable help in the CBA cytokine analyses. Drs M. Kaplan, D. Godfrey, J. Berzofsky, and M. Terabe provided valuable suggestions. Ms B. E. Juliar performed the statistical analysis of our data. The allophycocyanin-labeled CD1d tetramer loaded with the α-galactosylceramide analog PBS-57 was provided by the NIH Tetramer Facility (Emory University Vaccine Center, Atlanta, GA). We thank the members of the Flow Cytometry Resource Facility, Indiana University School of Medicine for their invaluable assistance in the electronic sorting of NKT cells for this study.
This work was supported by funding from NIH grants (R01 CA89026 and AI46455) and the Walther Cancer Institute to R.R.B., and NSF CHE-0194682 from the National Science Foundation to J.G.H. G.J.R. was supported by NIH training grant T32 DK007519. R.R.B. is a Scholar of the Leukemia & Lymphoma Society.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.J.R. performed research, analyzed, and interpreted data, and wrote the paper; M.A.K. and M.V. assisted with animal studies; W.D. and J.G.-H. contributed resources; R.R.B. conceptualized the work, analyzed and interpreted the data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randy R. Brutkiewicz, PhD, Department of Microbiology and Immunology, Indiana University School of Medicine, Building R2, Room 302, 950 W Walnut St, Indianapolis, IN 46202-5181; e-mail: rbrutkie@iupui.edu.
References
- 1.Shukla NN, Trippett TM. Non-Hodgkin's lymphoma in children and adolescents. Curr Oncol Rep. 2006;8:387–394. doi: 10.1007/s11912-006-0062-0. [DOI] [PubMed] [Google Scholar]
- 2.Smyth MJ, Godfrey DI. NKT cells and tumor immunity–a double-edged sword. Nat Immunol. 2000;1:459–460. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 3.Brutkiewicz RR. CD1d ligands: the good, the bad, and the ugly. J Immunol. 2006;177:769–775. doi: 10.4049/jimmunol.177.2.769. [DOI] [PubMed] [Google Scholar]
- 4.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berzins SP, Smyth MJ, Godfrey DI. Working with NKT cells–pitfalls and practicalities. Curr Opin Immunol. 2005;17:448–454. doi: 10.1016/j.coi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brutkiewicz RR, Lin Y, Cho S, Hwang YK, Sriram V, Roberts TJ. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit Rev Immunol. 2003;23:403–419. doi: 10.1615/critrevimmunol.v23.i56.30. [DOI] [PubMed] [Google Scholar]
- 8.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 9.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 10.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Van Kaer L, Brutkiewicz RR. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer. 2006;118:3045–3053. doi: 10.1002/ijc.21764. [DOI] [PubMed] [Google Scholar]
- 12.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 13.Sriram V, Cho S, Li P, et al. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc Natl Acad Sci U S A. 2002;99:8197–8202. doi: 10.1073/pnas.122636199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraguchi K, Takahashi T, Nakahara F, et al. CD1d expression level in tumor cells is an important determinant for anti-tumor immunity by natural killer T cells. Leuk Lymphoma. 2006;47:2218–2223. doi: 10.1080/10428190600682688. [DOI] [PubMed] [Google Scholar]
- 15.Serafini P, De Santo C, Marigo I, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand α-galactosylceramide in cancer-bearing state mediated by CD11b+ Gr-1+ cells producing nitric oxide. Cancer Res. 2006;66:11441–11446. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]
- 18.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 19.Roberts TJ, Sriram V, Spence PM, et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168:5409–5414. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- 20.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs JA, Cho S, Roberts TJ, et al. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–10754. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 23.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brutkiewicz RR, Klaus SJ, Welsh RM. Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat Immun. 1992;11:203–214. [PubMed] [Google Scholar]
- 25.Porcelli SA, Modlin RL. The CD1 System: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 26.Carnaud C, Lee D, Donnars O, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 27.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Brutkiewicz RR, Sriram V. Natural killer T (NKT) cells and their role in antitumor immunity. Crit Rev Oncol Hematol. 2002;41:287–298. doi: 10.1016/s1040-8428(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 29.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 30.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart TJ, Smyth MJ, Fernando GJ, Frazer IH, Leggatt GR. Inhibition of early tumor growth requires Jα18-positive (natural killer T) cells. Cancer Res. 2003;63:3058–3060. [PubMed] [Google Scholar]
- 32.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 34.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 36.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 37.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karadimitris A, Gadola S, Altamirano M, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98:3294–3298. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kita H, Naidenko OV, Kronenberg M, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 40.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:181–192. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallevaey T, Fontaine J, Breuilh L, et al. Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun. 2007;75:2171–2180. doi: 10.1128/IAI.01178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 44.Farag SS, Caligiuri MA. Immunologic approaches to acute leukemia in the elderly. Semin Hematol. 2006;43:118–125. doi: 10.1053/j.seminhematol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Smyth MJ, Thia KYT, Street SEA, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato T, Kaneko S, Kimizuka R, Okuda K. Periodontopathic bacterial endotoxin-induced tumor necrosis factor α production was inhibited by exercise in mice. FEMS Immunol Med Microbiol. 2006;47:262–266. doi: 10.1111/j.1574-695X.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- 49.Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhodapkar KM, Cirignano B, Chamian F, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 51.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with α-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasunami Y, Kojo S, Kitamura H, et al. Vα14 NK T cell-triggered IFN-γ production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med. 2005;202:913–918. doi: 10.1084/jem.20050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jothy S. CD44 and its partners in metastasis. Clin Exp Metastasis. 2003;20:195–201. doi: 10.1023/a:1022931016285. [DOI] [PubMed] [Google Scholar]
- 55.Kofler DM, Mayr C, Wendtner CM. Current status of immunotherapy in B cell malignancies. Curr Drug Targets. 2006;7:1371–1374. doi: 10.2174/138945006778559120. [DOI] [PubMed] [Google Scholar]