Abstract
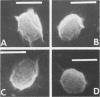
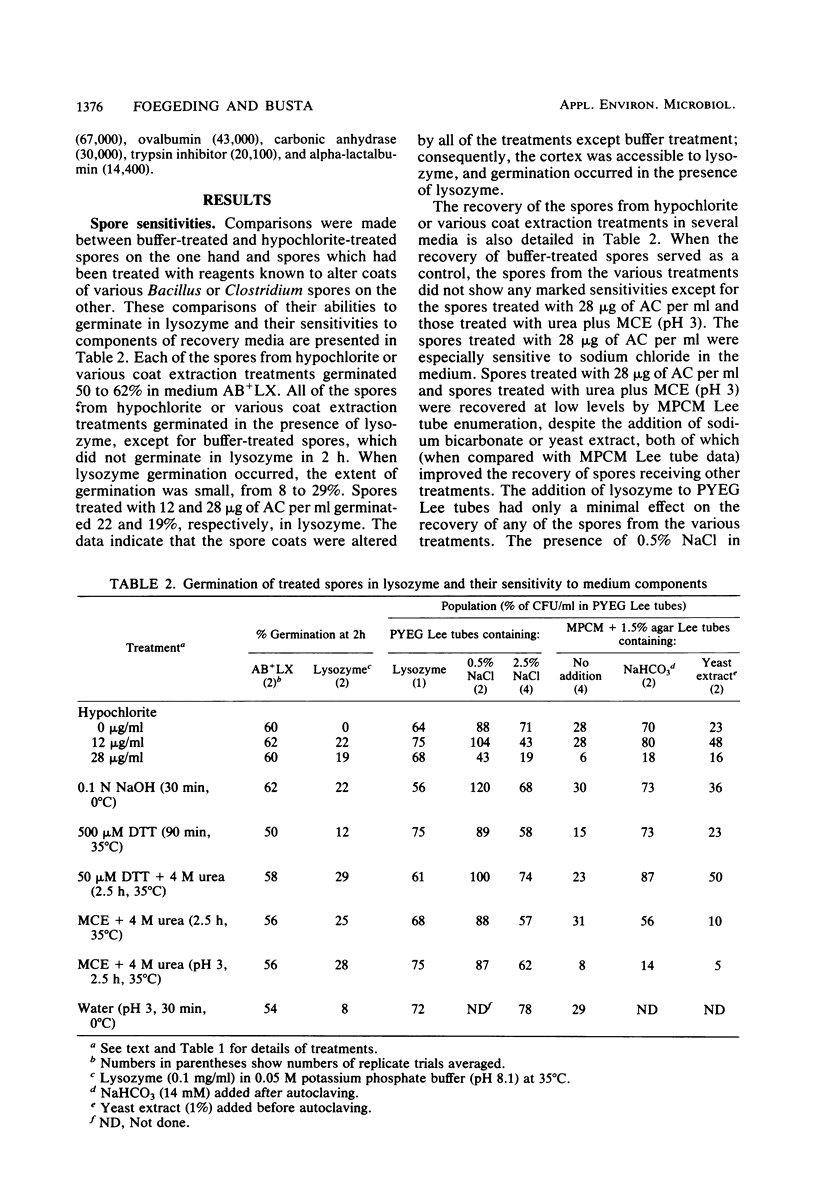
Hypochlorite-treated Clostridium botulinum 12885A spores, but not buffer-treated spores, could be germinated with lysozyme, indicating that their coats are made permeable to lysozyme by hypochlorite treatment so that the cortex is accessible. Hypochlorite-treated spores and spores extracted with 8 M urea-2-mercaptoethanol (pH 3.0) were sensitive to certain components of recovery media, but spores sensitized to lysozyme by other treatments were not. These data indicate that hypochlorite does more than increase coat permeability to lysozyme. Scanning electron microscopy revealed a more open-appearing surface of hypochlorite-treated spores, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated that a greater amount of protein was removed from hypochlorite-treated and other lysozyme-sensitized spores than from buffer-treated spores. The data suggest that spore coat proteins may be removed by hypochlorite treatment, and this may be responsible for the sensitivity of spores and for their observed ability to germinate in lysozyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderton G., Chen J. K., Ito K. A. Effect of lysozyne on the recovery of heated Clostridium botulinum spores. Appl Microbiol. 1974 Mar;27(3):613–615. doi: 10.1128/am.27.3.613-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Reconstitution of bacterial spore coat layers in vitro. J Bacteriol. 1971 Oct;108(1):571–578. doi: 10.1128/jb.108.1.571-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassier M., Ryter A. Sur un mutant de Clostridium perfringens donnant des spores sans tuniques à germination lysozyme-dépendante. Ann Inst Pasteur (Paris) 1971 Dec;121(6):717–732. [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegeding P. M., Busta F. F. Hypochlorite injury of Clostridium botulinum spores alters germination responses. Appl Environ Microbiol. 1983 Apr;45(4):1360–1368. doi: 10.1128/aem.45.4.1360-1368.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD G. W., HITCHINS A. D. SENSITIZATION OF BACTERIAL SPORES TO LYSOZYME AND TO HYDROGEN PEROXIDE WITH AGENTS WHICH RUPTURE DISULPHIDE BONDS. J Gen Microbiol. 1963 Dec;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- Greenberg R. A., Bladel B. O., Zingelmann W. J. Use of the anaerobic pouch in isolating Clostridium botulinum spores from fresh meats. Appl Microbiol. 1966 Mar;14(2):223–228. doi: 10.1128/am.14.2.223-228.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikovsky A., Pankratz H. S., Sadoff H. L. Ultrastructural and chemical changes in spores of Bacillus cereus after action of disinfectants. J Appl Bacteriol. 1975 Feb;38(1):39–46. doi: 10.1111/j.1365-2672.1975.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Labbe R. G., Reich R. R., Duncan C. L. Alteration in ultrastructure and germination of Clostridium perfringens type A spores following extraction of spore coats. Can J Microbiol. 1978 Dec;24(12):1526–1536. doi: 10.1139/m78-244. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ogg J. E., Lee S. Y., Ogg B. J. A modified tube method for the cultivation and enumeration of anaerobic bacteria. Can J Microbiol. 1979 Sep;25(9):987–990. doi: 10.1139/m79-151. [DOI] [PubMed] [Google Scholar]
- Sacks L. E. Influence of cations on lysozyme-induced germination of coatless spores of Clostridium perfringens 8-6. Biochim Biophys Acta. 1981 Apr 17;674(1):118–127. doi: 10.1016/0304-4165(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L. R., Waites W. M. The effect of chlorine on spores of Clostridium bifermentans, Bacillus subtilis and Bacillus cereus. J Gen Microbiol. 1975 Aug;89(2):337–344. doi: 10.1099/00221287-89-2-337. [DOI] [PubMed] [Google Scholar]
- Wyatt L. R., Waites W. M. The effect of hypochlorite on the germination of spores of Clostridium bifermentans. J Gen Microbiol. 1973 Oct;78(2):383–385. doi: 10.1099/00221287-78-2-383. [DOI] [PubMed] [Google Scholar]
- Wyatt L. R., Waites W. M. The effect of sodium hydroxide or dithiothreitol-urea on spores of germination mutants of Clostridium bifermentans. J Gen Microbiol. 1974 Oct;84(2):391–394. doi: 10.1099/00221287-84-2-391. [DOI] [PubMed] [Google Scholar]