Abstract
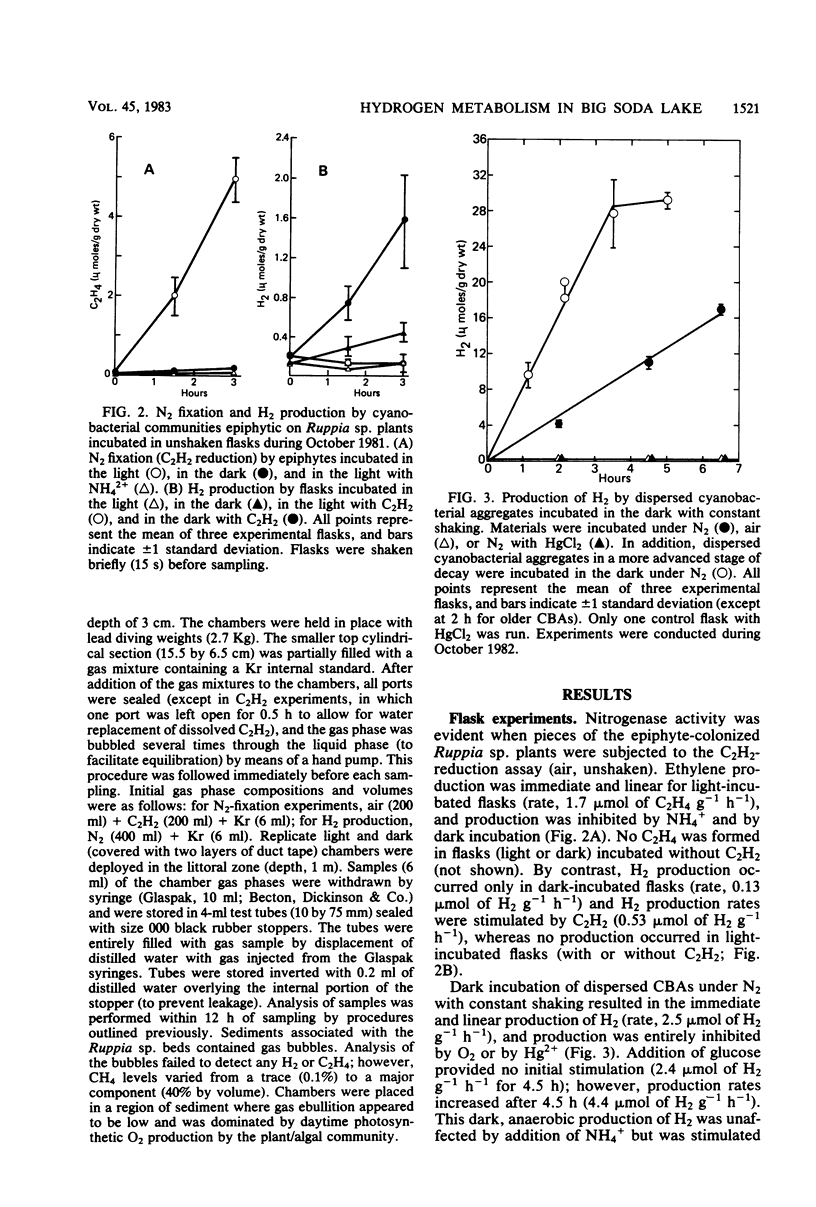
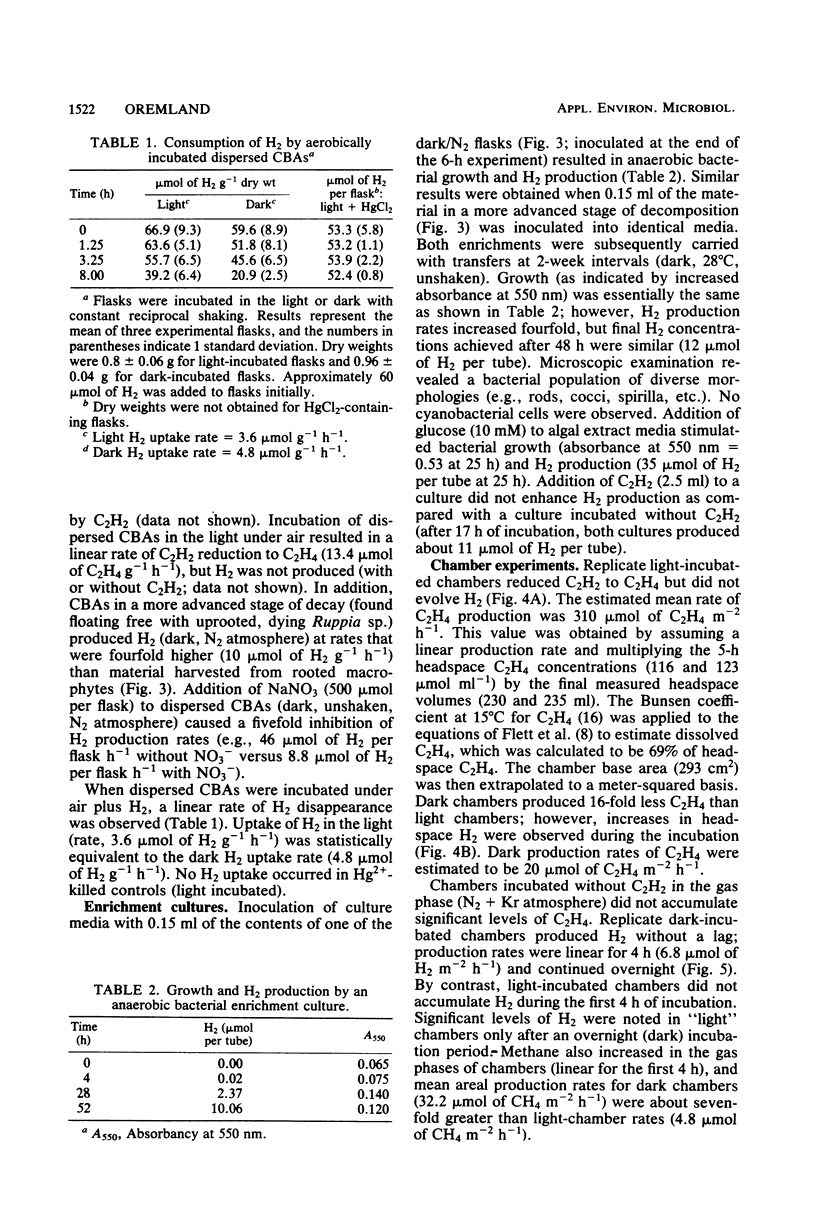
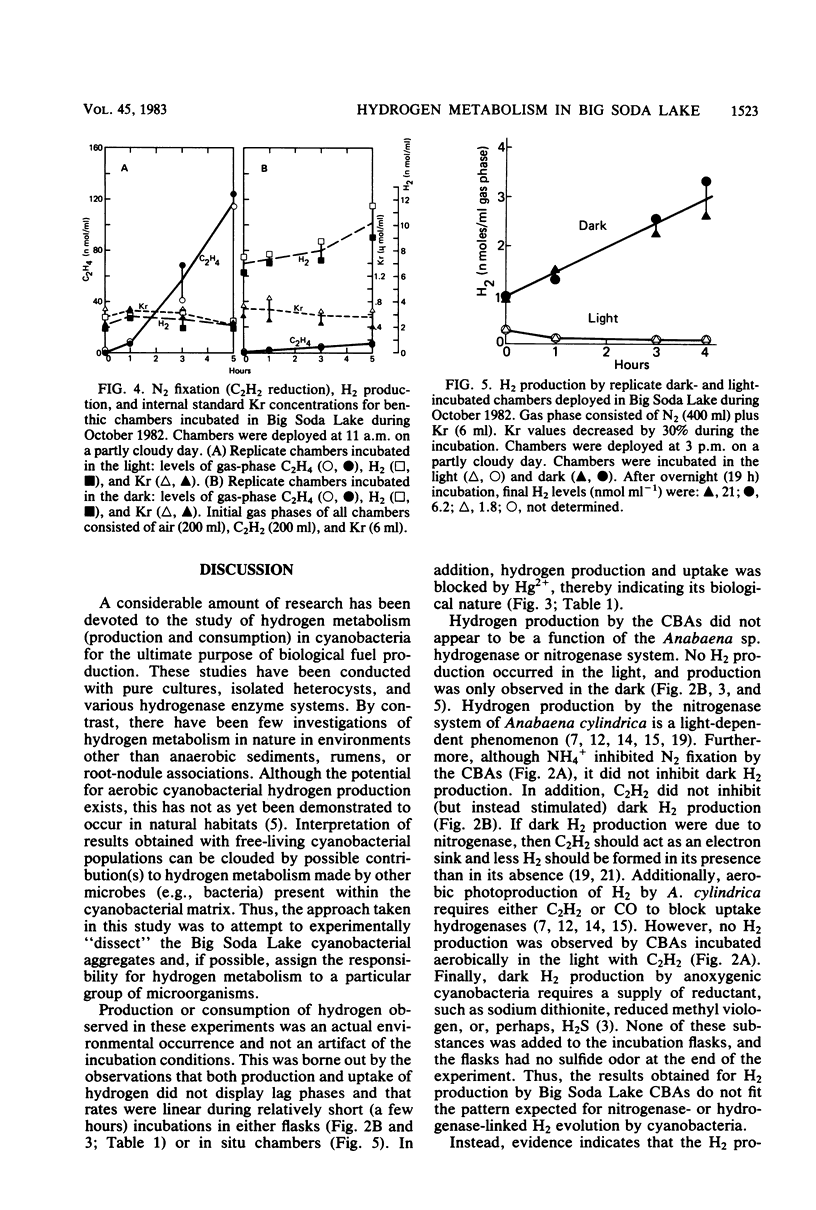
Hydrogen production by incubated cyanobacterial epiphytes occurred only in the dark, was stimulated by C2H2, and was inhibited by O2. Addition of NO3− inhibited dark, anaerobic H2 production, whereas the addition of NH4+ inhibited N2 fixation (C2H2 reduction) but not dark H2 production. Aerobically incubated cyanobacterial aggregates consumed H2, but light-incubated rates (3.6 μmol of H2 g−1 h−1) were statistically equivalent to dark uptake rates (4.8 μmol of H2 g−1 h−1), which were statistically equivalent to dark, anaerobic production rates (2.5 to 10 μmol of H2 g−1 h−1). Production rates of H2 were fourfold higher for aggregates in a more advanced stage of decomposition. Enrichment cultures of H2-producing fermentative bacteria were recovered from freshly harvested, H2-producing cyanobacterial aggregates. Hydrogen production in these cyanobacterial communities appears to be caused by the resident bacterial flora and not by the cyanobacteria. In situ areal estimates of dark H2 production by submerged epiphytes (6.8 μmol of H2 m−2 h−1) were much lower than rates of light-driven N2 fixation by the epiphytic cyanobacteria (310 μmol of C2H4 m−2 h−1).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belkin S., Padan E. Hydrogen metabolism in the facultative anoxygenic cyanobacteria (blue-green algae) Oscillatoria limnetica and Aphanothece halophytica. Arch Microbiol. 1978 Jan 23;116(1):109–111. doi: 10.1007/BF00408741. [DOI] [PubMed] [Google Scholar]
- Conrad R., Aragno M., Seiler W. Production and consumption of hydrogen in a eutrophic lake. Appl Environ Microbiol. 1983 Feb;45(2):502–510. doi: 10.1128/aem.45.2.502-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daday A., Platz R. A., Smith G. D. Anaerobic and aerobic hydrogen gas formation by the blue-green alga Anabaena cylindrica. Appl Environ Microbiol. 1977 Nov;34(5):478–483. doi: 10.1128/aem.34.5.478-483.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Light and dark reactions of the uptake hydrogenase in anabaena 7120. Plant Physiol. 1981 Sep;68(3):712–716. doi: 10.1104/pp.68.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Physiological reactions of the reversible hydrogenase from anabaena 7120. Plant Physiol. 1981 Sep;68(3):717–721. doi: 10.1104/pp.68.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Daday A., Smith G. D. Duration of Hydrogen Formation by Anabaena cylindrica B629 in Atmospheres of Argon, Air, and Nitrogen. Appl Environ Microbiol. 1979 Sep;38(3):530–536. doi: 10.1128/aem.38.3.530-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Daday A., Smith G. D. Effects of Ammonium Ions, Oxygen, Carbon Monoxide, and Acetylene on Anaerobic and Aerobic Hydrogen Formation by Anabaena cylindrica B629. Appl Environ Microbiol. 1979 Sep;38(3):521–529. doi: 10.1128/aem.38.3.521-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Marsh L., Desmarais D. J. Methanogenesis in big soda lake, nevada: an alkaline, moderately hypersaline desert lake. Appl Environ Microbiol. 1982 Feb;43(2):462–468. doi: 10.1128/aem.43.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Microbial formation of ethane in anoxic estuarine sediments. Appl Environ Microbiol. 1981 Jul;42(1):122–129. doi: 10.1128/aem.42.1.122-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ortiz J. M., Burris R. H. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975 Aug;123(2):537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. A., Hill S., Yates M. G. Inhibition by acetylene of conventional hydrogenase in nitrogen-fixing bacteria. Nature. 1976 Jul 15;262(5565):209–210. doi: 10.1038/262209a0. [DOI] [PubMed] [Google Scholar]
- Triska F. J., Oremland R. S. Denitrification associated with periphyton communities. Appl Environ Microbiol. 1981 Oct;42(4):745–748. doi: 10.1128/aem.42.4.745-748.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


