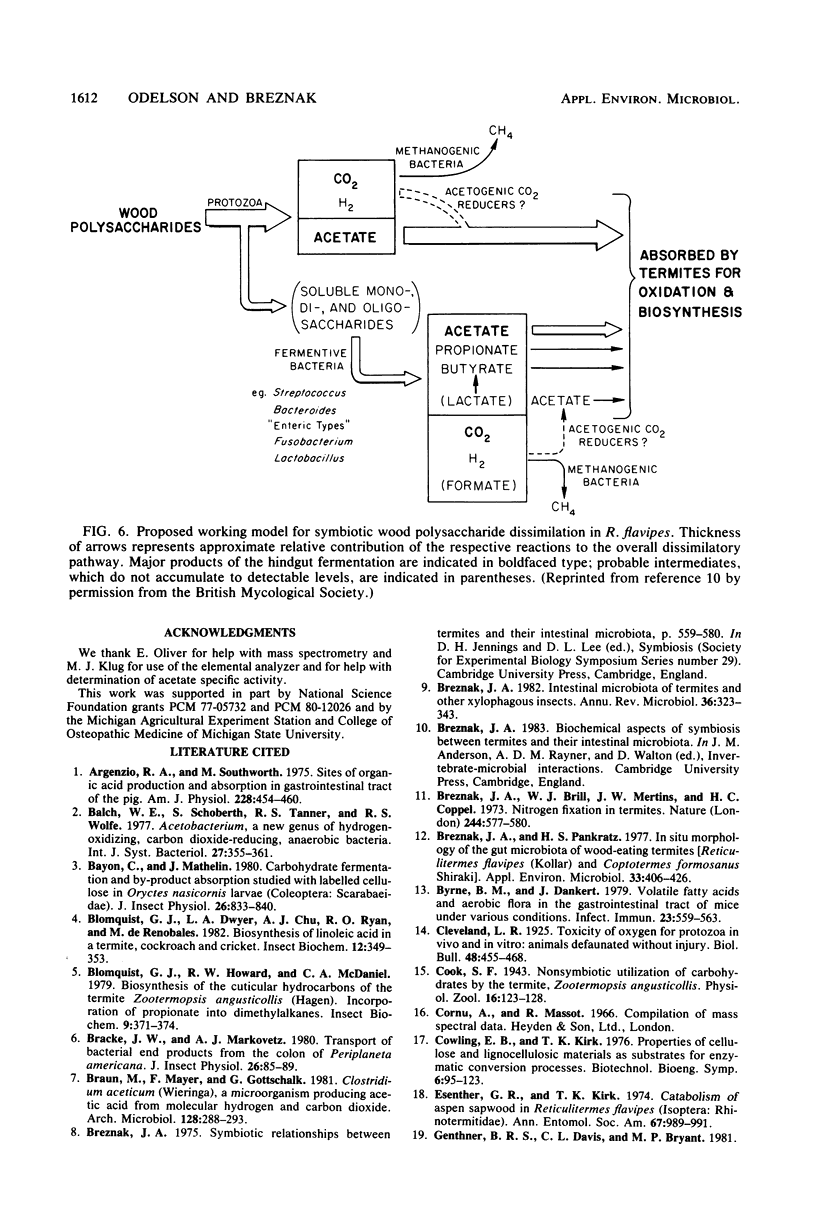
Abstract
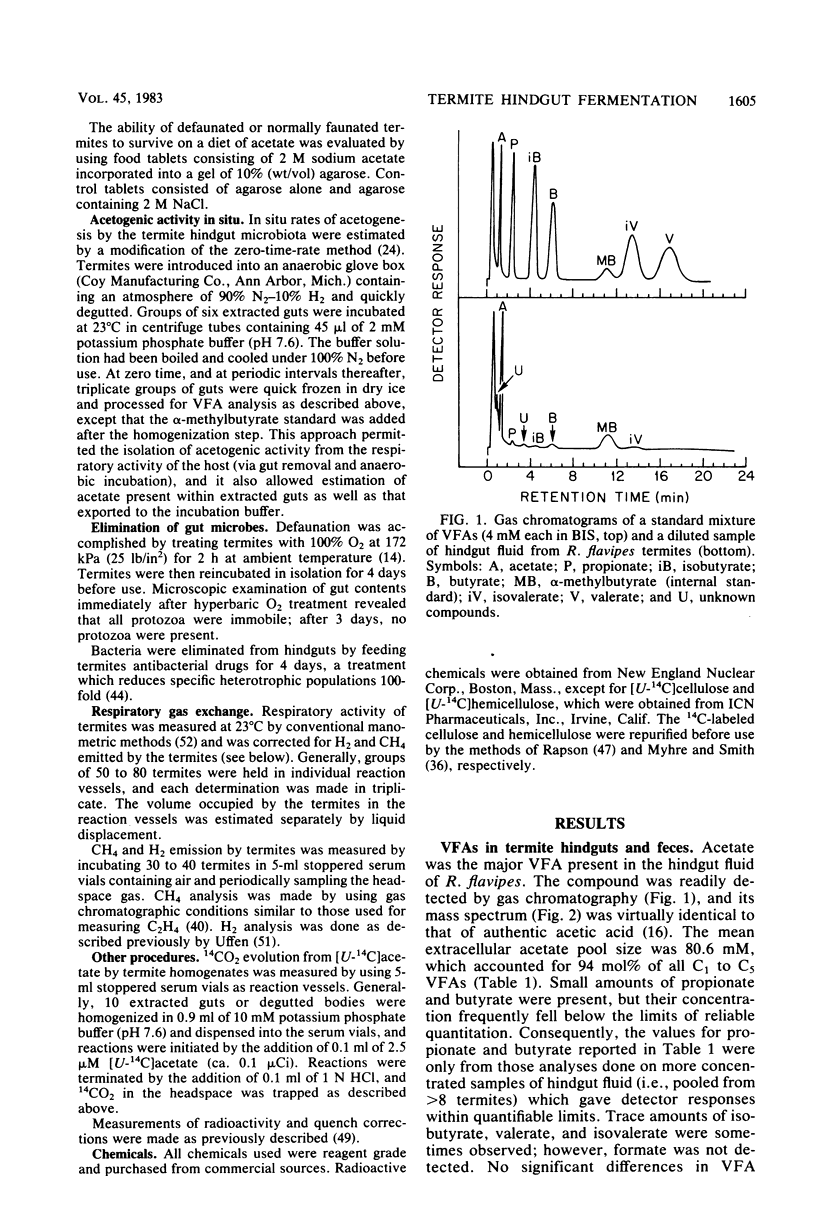
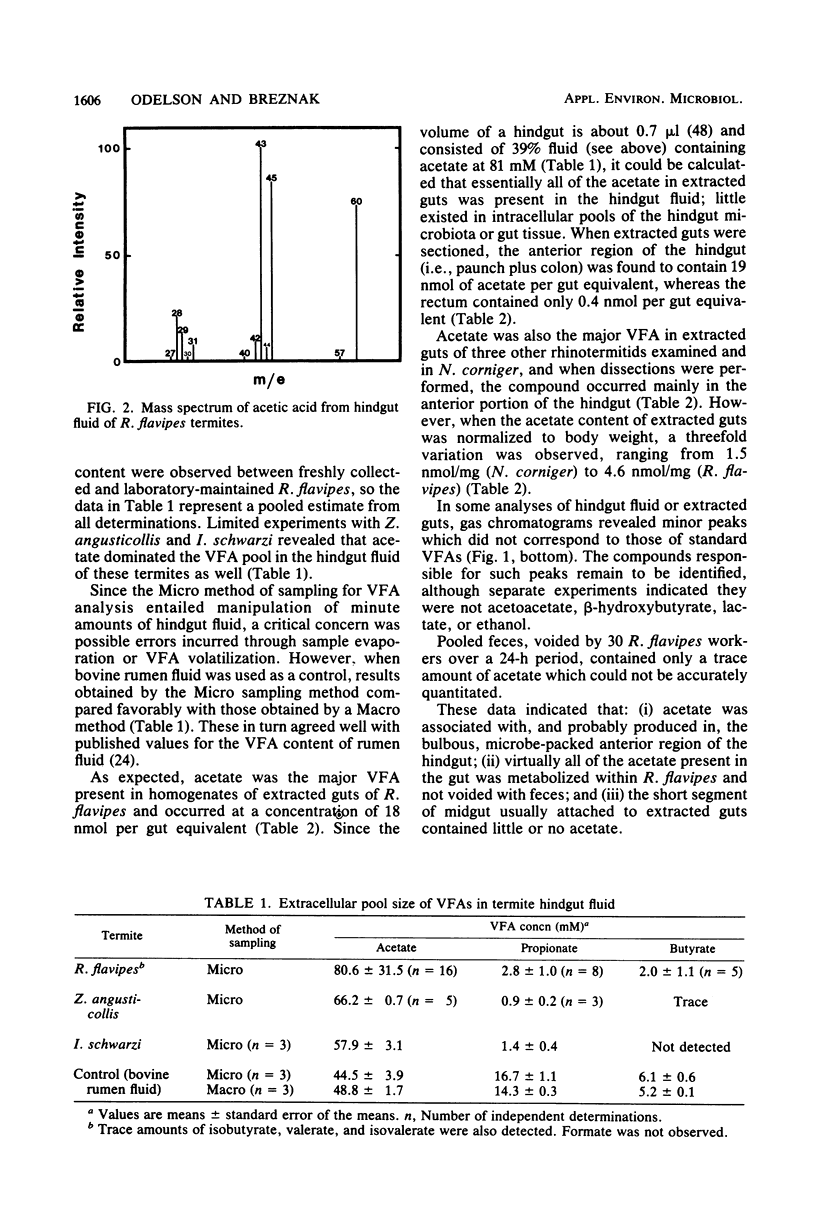
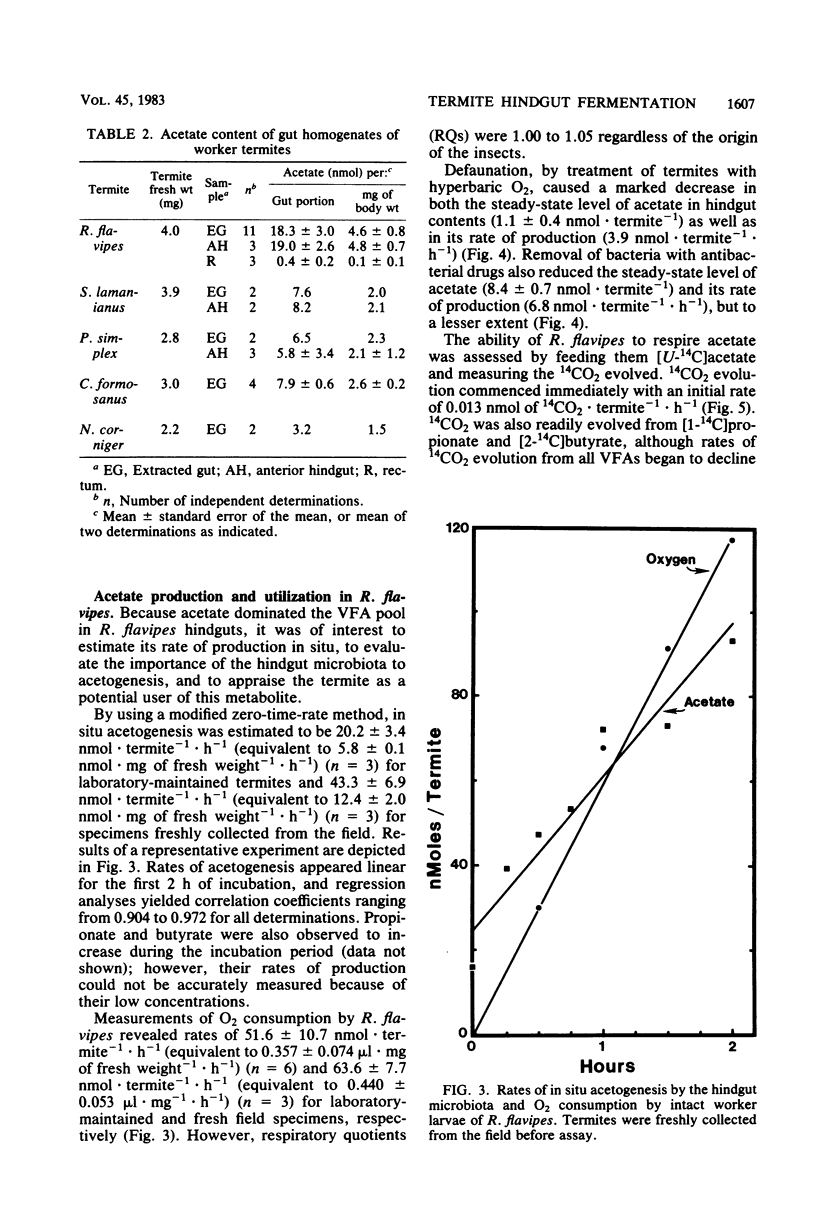
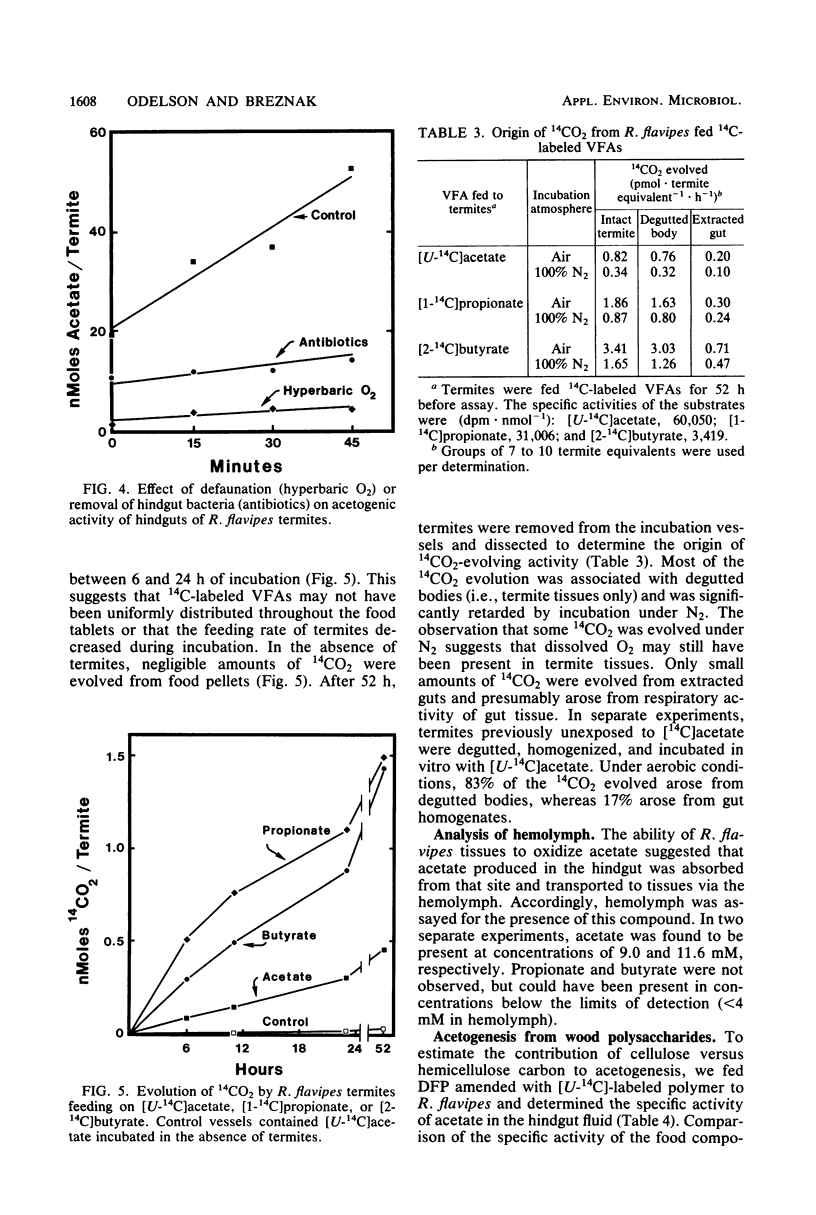
Acetate dominated the extracellular pool of volatile fatty acids (VFAs) in the hindgut fluid of Reticulitermes flavipes, Zootermopsis angusticollis, and Incisitermes schwarzi, where it occurred at concentrations of 57.9 to 80.6 mM and accounted for 94 to 98 mol% of all VFAs. Small amounts of C3 to C5 VFAs were also observed. Acetate was also the major VFA in hindgut homogenates of Schedorhinotermes lamanianus, Prorhinotermes simplex, Coptotermes formosanus, and Nasutitermes corniger. Estimates of in situ acetogenesis by the hindgut microbiota of R. flavipes (20.2 to 43.3 nmol · termite−1 · h−1) revealed that this activity could support 77 to 100% of the respiratory requirements of the termite (51.6 to 63.6 nmol of O2 · termite−1 · h−1). This conclusion was buttressed by the demonstration of acetate in R. flavipes hemolymph (at 9.0 to 11.6 mM), but not in feces, and by the ability of termite tissues to readily oxidize acetate to CO2. About 85% of the acetate produced by the hindgut microbiota was derived from cellulose C; the remainder was derived from hemicellulose C. Selective removal of major groups of microbes from the hindgut of R. flavipes indicated that protozoa were primarily responsible for acetogenesis but that bacteria also functioned in this capacity. H2 and CH4 were evolved by R. flavipes (usually about 0.4 nmol · termite−1 · h−1), but these compounds represented a minor fate of electrons derived from wood dissimilation within R. flavipes. A working model is proposed for symbiotic wood polysaccharide degradation in R. flavipes, and the possible roles of individual gut microbes, including CO2-reducing acetogenic bacteria, are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenzio R. A., Southworth M. Sites of organic acid production and absorption in gastrointestinal tract of the pig. Am J Physiol. 1975 Feb;228(2):454–460. doi: 10.1152/ajplegacy.1975.228.2.454. [DOI] [PubMed] [Google Scholar]
- Braun M., Mayer F., Gottschalk G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol. 1981 Jan;128(3):288–293. doi: 10.1007/BF00422532. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Brill W. J., Mertins J. W., Coppel H. C. Nitrogen fixation in termites. Nature. 1973 Aug 31;244(5418):577–580. doi: 10.1038/244577a0. [DOI] [PubMed] [Google Scholar]
- Breznak J. A. Intestinal microbiota of termites and other xylophagous insects. Annu Rev Microbiol. 1982;36:323–343. doi: 10.1146/annurev.mi.36.100182.001543. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Pankratz H. S. In situ morphology of the gut microbiota of wood-eating termites [Reticulitermes flavipes (Kollar) and Coptotermes formosanus Shiraki]. Appl Environ Microbiol. 1977 Feb;33(2):406–426. doi: 10.1128/aem.33.2.406-426.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A. Symbiotic relationships between termites and their intestinal microbiota. Symp Soc Exp Biol. 1975;(29):559–580. [PubMed] [Google Scholar]
- Byrne B. M., Dankert J. Volatile fatty acids and aerobic flora in the gastrointestinal tract of mice under various conditions. Infect Immun. 1979 Mar;23(3):559–563. doi: 10.1128/iai.23.3.559-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling E. B., Kirk T. K. Properties of cellulose and lignocellulosic materials as substrates for enzymatic conversion processes. Biotechnol Bioeng Symp. 1976;(6):95–123. [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor J. Etude radiographique du transit intestinal chez un Termite supérieur. Experientia. 1967 Oct 15;23(10):820–821. doi: 10.1007/BF02146863. [DOI] [PubMed] [Google Scholar]
- Kovoor J. Présence d'acides gras volatils dans la panse d'un termite supérieur (Microcerotermes edentatus Was., Amitermitinae) C R Acad Sci Hebd Seances Acad Sci D. 1967 Jan 16;264(3):486–488. [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., James W. P. Short chain fatty acid absorption by the human large intestine. Gut. 1978 Sep;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Anaerobic degradation of uric Acid by gut bacteria of termites. Appl Environ Microbiol. 1980 Jul;40(1):125–132. doi: 10.1128/aem.40.1.125-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Gut bacteria recycle uric acid nitrogen in termites: A strategy for nutrient conservation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4601–4605. doi: 10.1073/pnas.78.7.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Nitrogen-fixing Enterobacter agglomerans isolated from guts of wood-eating termites. Appl Environ Microbiol. 1977 Feb;33(2):392–399. doi: 10.1128/aem.33.2.392-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Uric Acid-Degrading Bacteria in Guts of Termites [Reticulitermes flavipes (Kollar)]. Appl Environ Microbiol. 1980 Jul;40(1):117–124. doi: 10.1128/aem.40.1.117-124.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Breznak J. A. Cross-Feeding of Lactate Between Streptococcus lactis and Bacteroides sp. Isolated from Termite Hindguts. Appl Environ Microbiol. 1979 Jun;37(6):1206–1210. doi: 10.1128/aem.37.6.1206-1210.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Breznak J. A. Heterotrophic bacteria present in hindguts of wood-eating termites [Reticulitermes flavipes (Kollar)]. Appl Environ Microbiol. 1978 May;35(5):930–936. doi: 10.1128/aem.35.5.930-936.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin M. A. Cellulose Metabolism by the Termite Flagellate Trichomitopsis termopsidis. Appl Environ Microbiol. 1980 Apr;39(4):859–863. doi: 10.1128/aem.39.4.859-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin M. A. Cellulose metabolism by the flagellate trichonympha from a termite is independent of endosymbiotic bacteria. Science. 1981 Jan 2;211(4477):58–59. doi: 10.1126/science.211.4477.58. [DOI] [PubMed] [Google Scholar]
- Zimmerman P. R., Greenberg J. P., Wandiga S. O., Crutzen P. J. Termites: a potentially large source of atmospheric methane, carbon dioxide, and molecular hydrogen. Science. 1982 Nov 5;218(4572):563–565. doi: 10.1126/science.218.4572.563. [DOI] [PubMed] [Google Scholar]


