Abstract
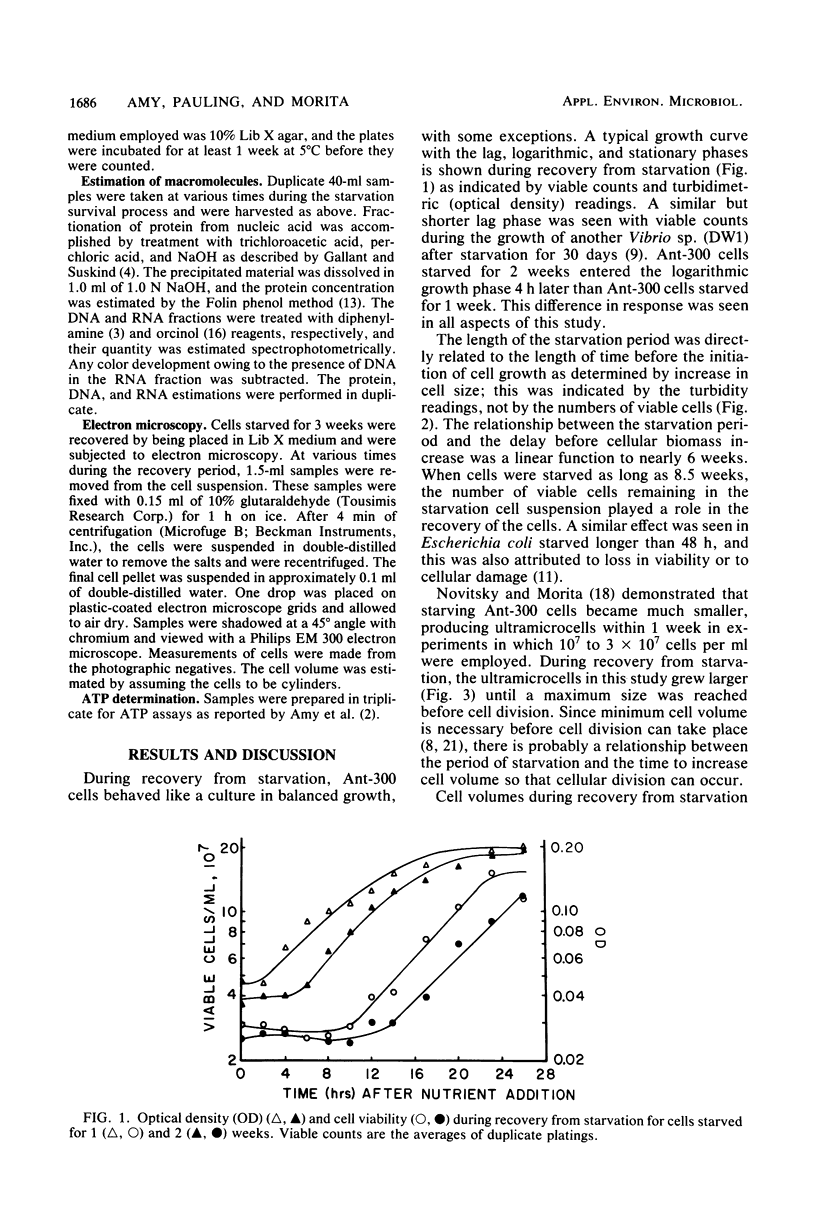
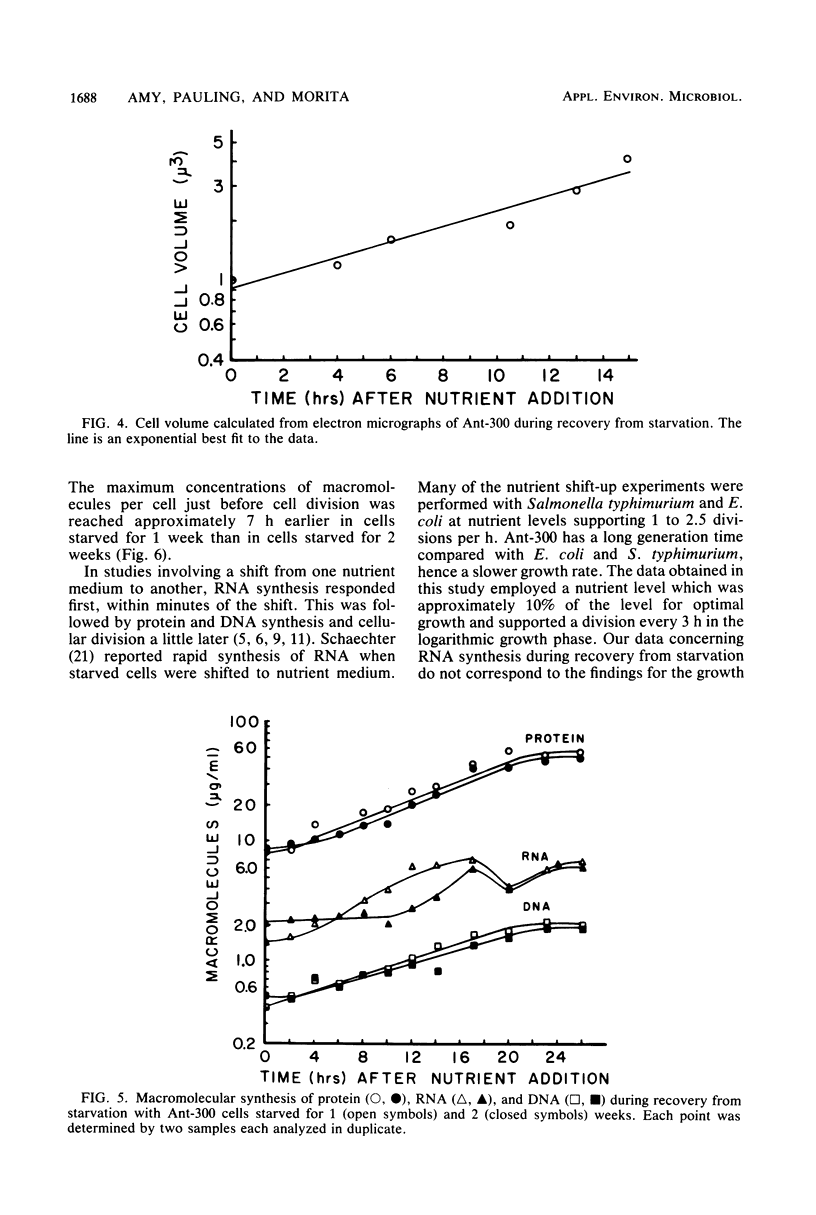
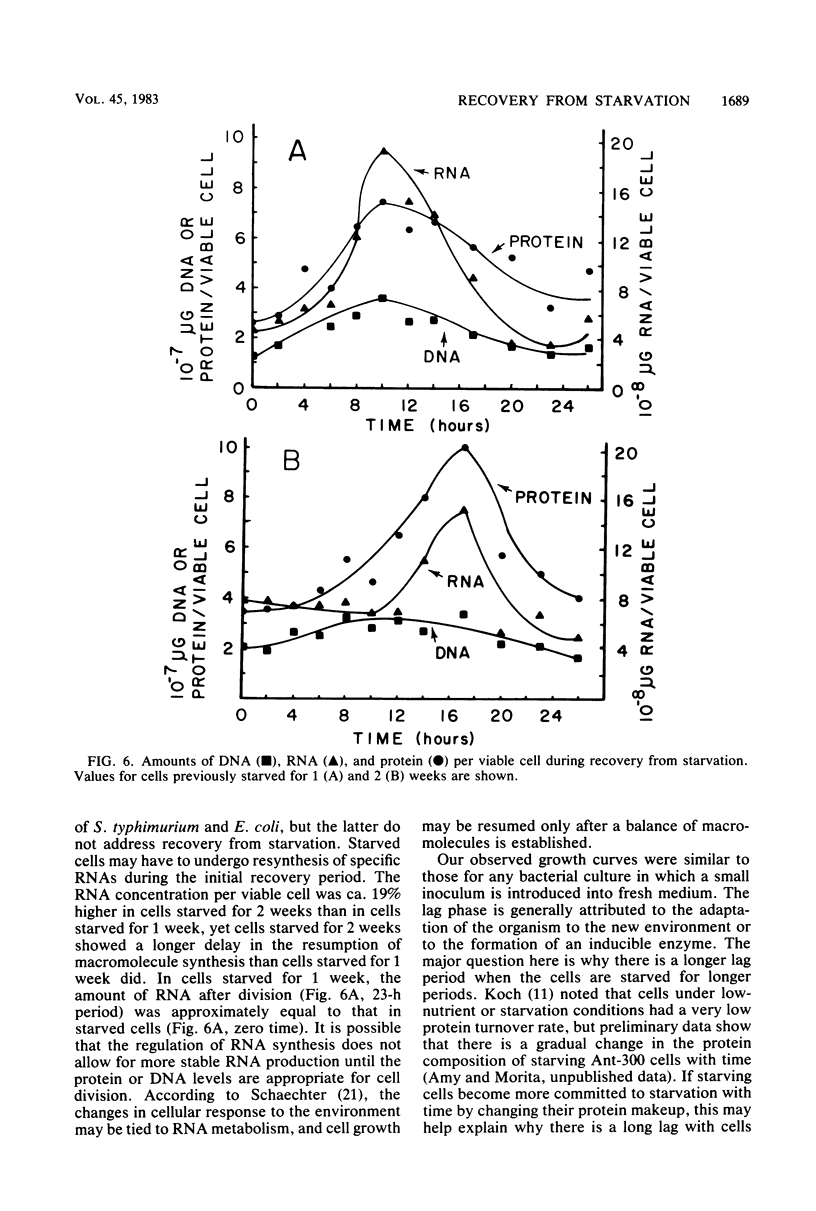
A marine psychrophilic Vibrio sp., Ant-300, recovered from starvation after the addition of 1 volume of complete nutrient medium to 9 volumes of starvation menstruum. Turbidity (measured by optical density), viable cell counts, cell size (measured from electron micrographs), and cellular concentrations of protein, DNA, and RNA were monitored with recovery time. The usual growth curve of bacterial cultures was observed. On a per viable cell basis, protein, DNA, and RNA increased to maximum values just before cell division and then returned to close to the initial starved-cell value during the stationary phase. Cells under complete starvation conditions or missing only one nutrient in the stationary phase responded with cell division resulting in many smaller cells. The length of the lag phase during recovery was directly proportional to the length of the prior starvation period, even when identical numbers of cells were used for recovery. Cells appeared to pass more deeply into dormancy with starvation time.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT J., SUSKIND S. R. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J Bacteriol. 1961 Aug;82:187–194. doi: 10.1128/jb.82.2.187-194.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W. Growth characteristics of heterotrophic bacteria in seawater. J Bacteriol. 1968 Feb;95(2):722–723. doi: 10.1128/jb.95.2.722-723.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJELDGAARD N. O., MAALOE O., SCHAECHTER M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- KJELDGAARD N. O. The kinetics of ribonucleic acid- and protein formation in Salmonella typhimurium during the transition between different states of balance growth. Biochim Biophys Acta. 1961 Apr 29;49:64–76. doi: 10.1016/0006-3002(61)90870-8. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M. Patterns of cellular control during unbalanced growth. Cold Spring Harb Symp Quant Biol. 1961;26:53–62. doi: 10.1101/sqb.1961.026.01.011. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



