Abstract
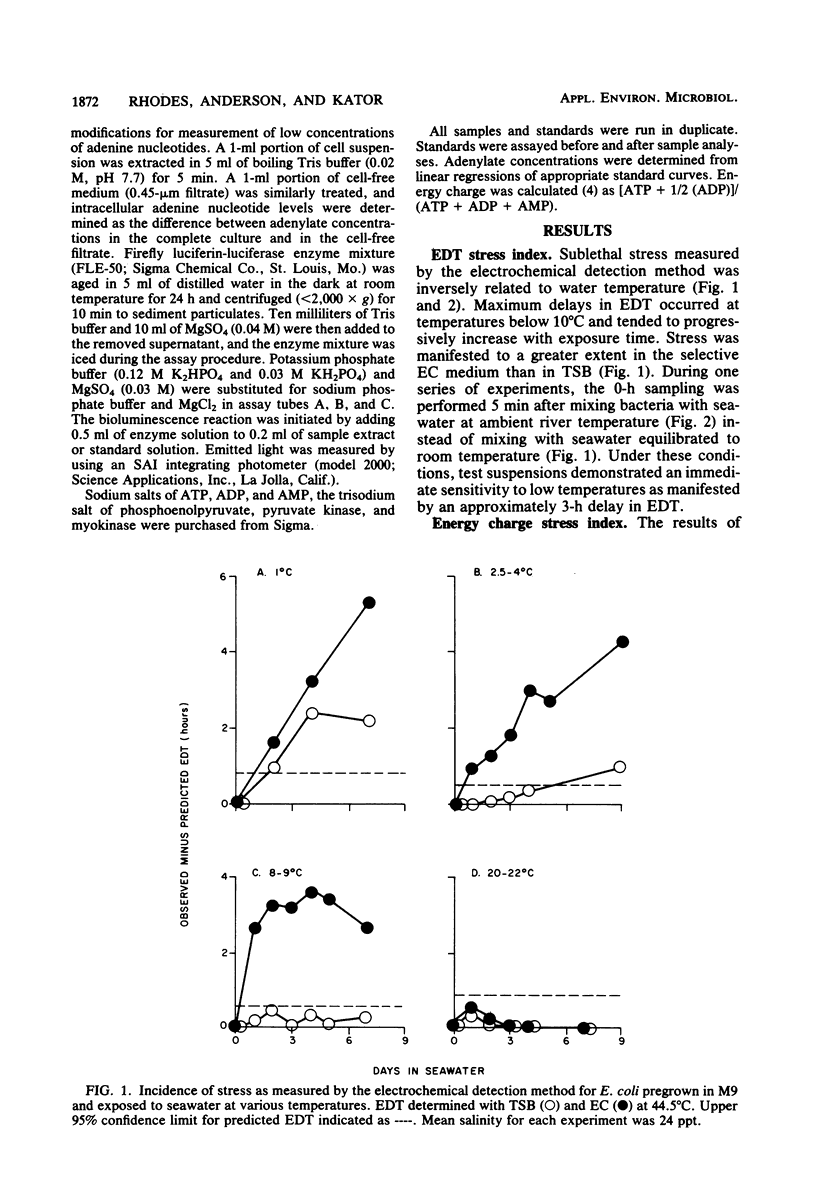
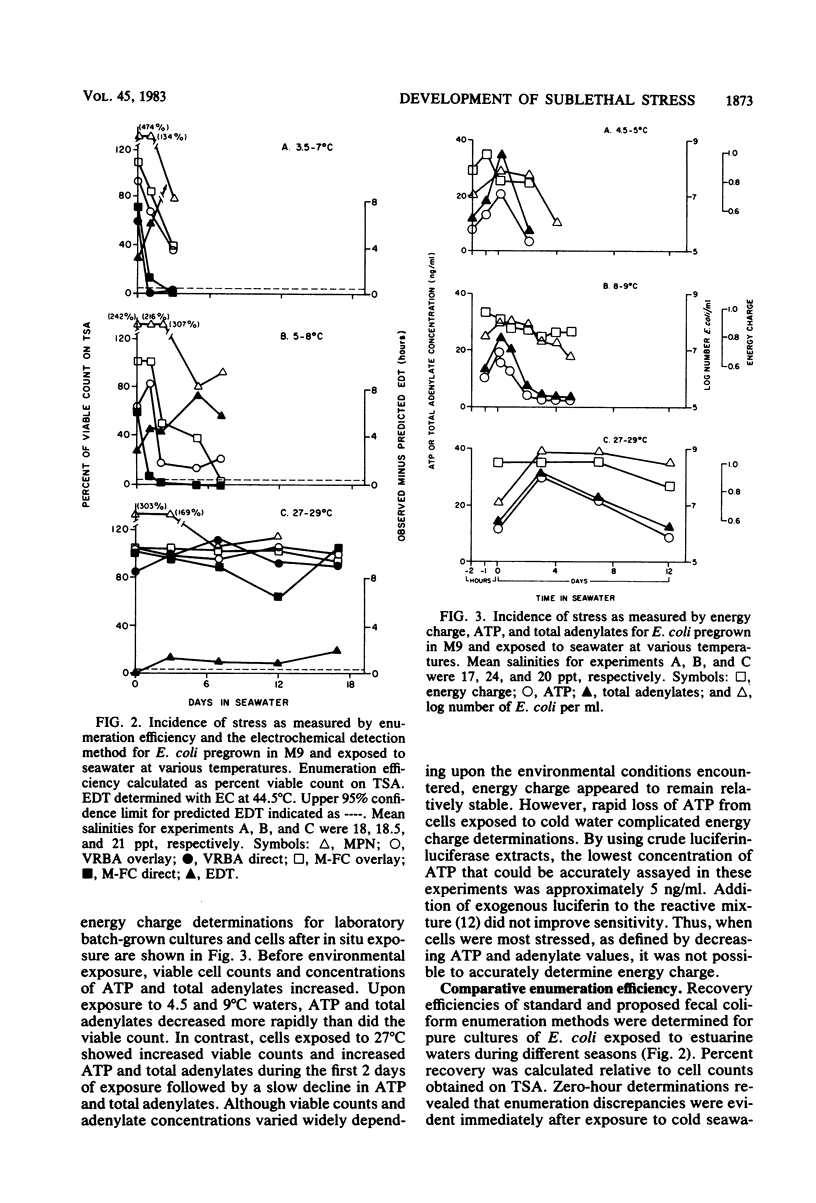
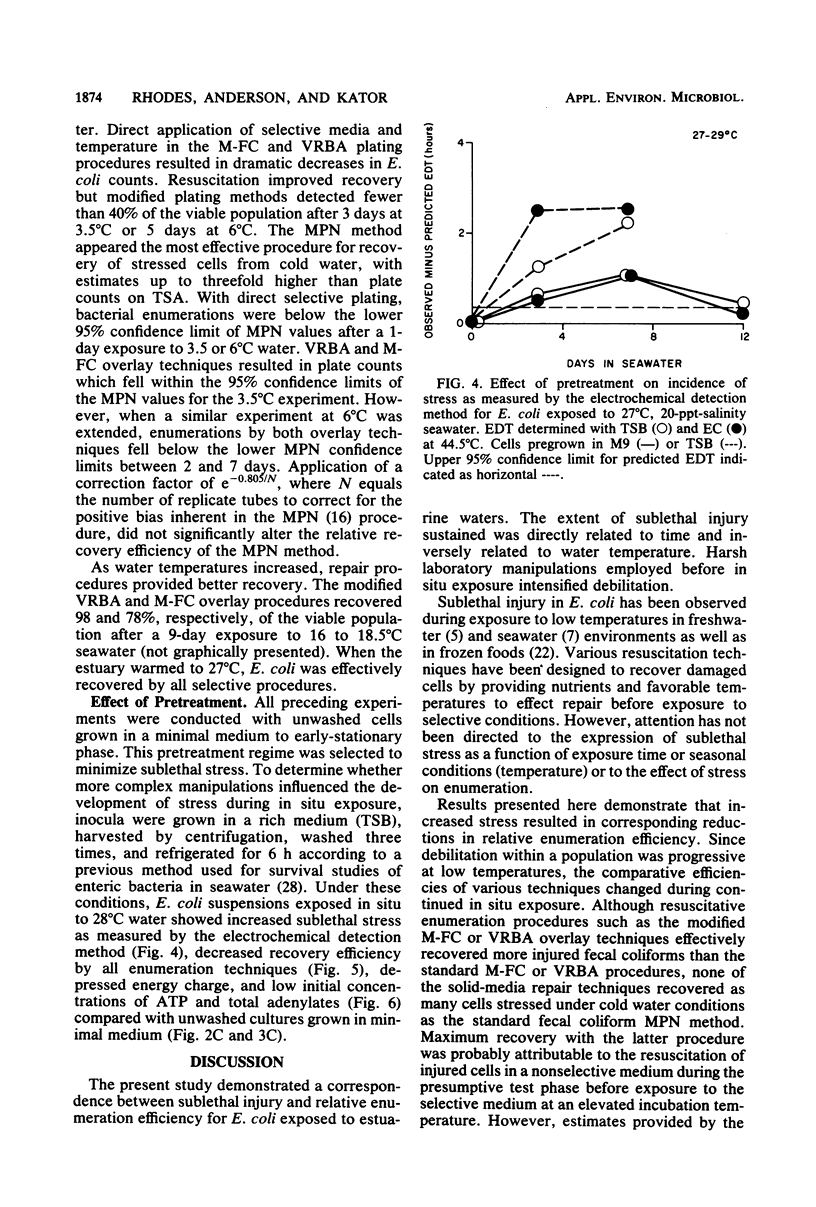
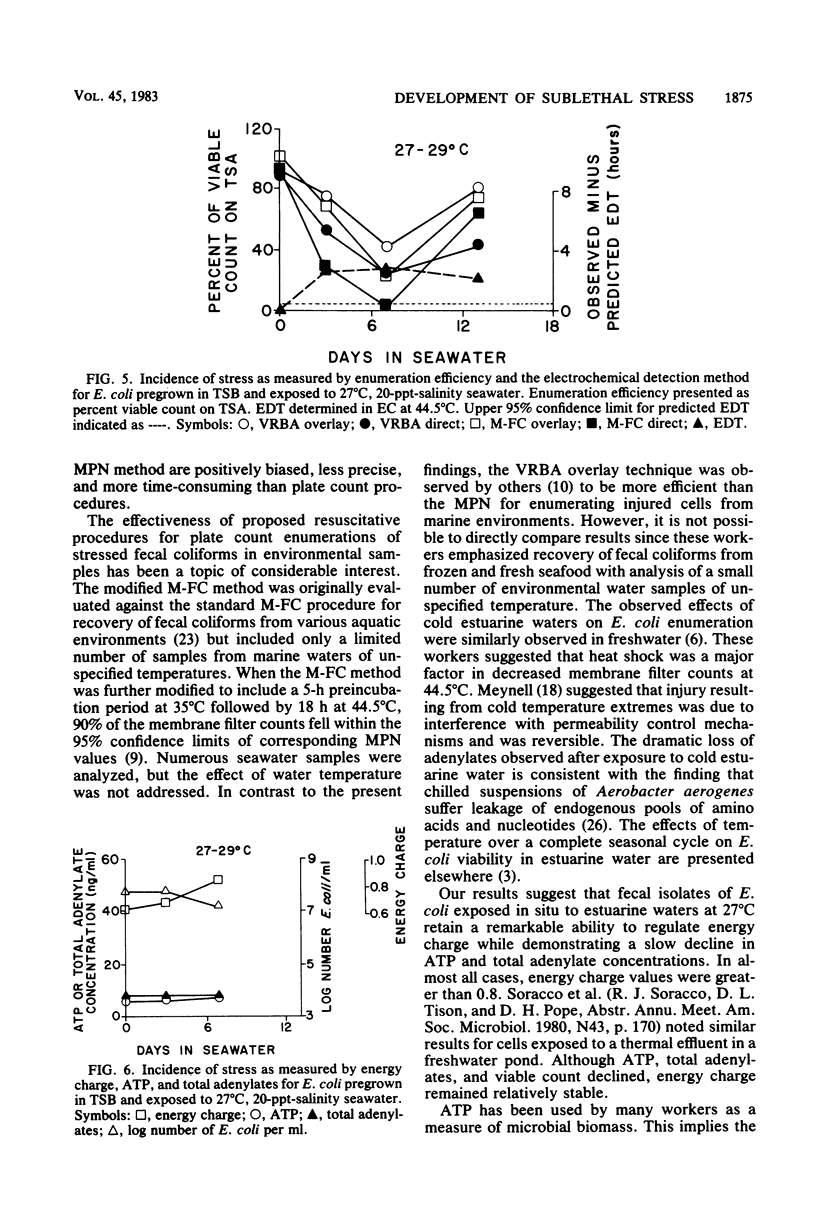
Development of sublethal stress in Escherichia coli exposed in situ to estuarine waters was examined during various seasons. An electrochemical detection technique was utilized to derive a stress index based upon the difference between a predicted electrochemical response time in Trypticase soy broth or EC medium at 44.5 degrees C estimated from a standard curve for unstressed cells and an observed response time for cells exposed to seawater. This stress index was related to recovery efficiencies of seawater-exposed cells, using a variety of standard and resuscitative enumeration procedures. Stress was further studied by determination of the adenylate energy charge. Sublethal stress as measured by the electrochemical detection method was an inverse function of water temperature, with maximum stress occurring after exposure to temperatures below 10 degrees C. Total adenylates and ATP decreased dramatically at low temperatures, although energy charge remained relatively constant under various environmental conditions. Decreases in E. coli ATP suggest that ATP may not be an adequate measure of biomass for in situ stressed cells. Discrepancies in enumeration efficiency were most pronounced at temperatures below 10 degrees C. Resuscitative procedures for solid-media techniques increased the recovery of stressed cells under cold water conditions but were not as effective as the standard most-probable-number procedure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Rhodes M. W., Kator H. I. Seasonal variation in survival of Escherichia coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol. 1983 Jun;45(6):1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson I. C., Rhodes M., Kator H. Sublethal stress in Escherichia coli: a function of salinity. Appl Environ Microbiol. 1979 Dec;38(6):1147–1152. doi: 10.1128/aem.38.6.1147-1152.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport C. V., Sparrow E. B., Gordon R. C. Fecal indicator bacteria persistence under natural conditions in an ice-covered river. Appl Environ Microbiol. 1976 Oct;32(4):527–536. doi: 10.1128/aem.32.4.527-536.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe L. L., Penrose W. R. "Bactericidal" property of seawater: death or debilitation? Appl Environ Microbiol. 1978 May;35(5):829–833. doi: 10.1128/aem.35.5.829-833.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A. P., Strickland E. R., Cabelli V. J. Membrane filter method for enumerating Escherichia coli. Appl Environ Microbiol. 1981 May;41(5):1152–1158. doi: 10.1128/aem.41.5.1152-1158.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney C. R., Ray B., Speck M. L. Repair detection procedure for enumeration of fecal coliforms and enterococci from seafoods and marine environments. Appl Environ Microbiol. 1979 May;37(5):947–953. doi: 10.1128/aem.37.5.947-953.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. A., Wu S. Stress: a factor to be considered in heterotrophic microorganism enumeration from aquatic environments. Appl Microbiol. 1974 Feb;27(2):429–431. doi: 10.1128/am.27.2.429-431.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G. The effect of sudden chilling on Escherichia coli. J Gen Microbiol. 1958 Oct;19(2):380–389. doi: 10.1099/00221287-19-2-380. [DOI] [PubMed] [Google Scholar]
- McCARTHY J. A., THOMAS H. A., Jr, DELANEY J. E. Evaluation of the reliability of coliform density tests. Am J Public Health Nations Health. 1958 Dec;48(12):1628–1635. doi: 10.2105/ajph.48.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- Olson B. H. Enchanced accuracy of coliform testing in seawater by a modification of the most-probable-number method. Appl Environ Microbiol. 1978 Sep;36(3):438–444. doi: 10.1128/aem.36.3.438-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E., Geldreich E. E., Litsky W. Improved membrane filter method for fecal coliform analysis. Appl Microbiol. 1975 Apr;29(4):532–536. doi: 10.1128/am.29.4.532-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Effect of chilling on Aerobacter aerogenes in aqueous suspension. J Gen Microbiol. 1962 Dec;29:719–730. doi: 10.1099/00221287-29-4-719. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., McFeters G. A., Schillinger J. E. Membrane filter technique for the quantification of stressed fecal coliforms in the aquatic environment. Appl Environ Microbiol. 1977 Jul;34(1):42–46. doi: 10.1128/aem.34.1.42-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos G. J., Swartz R. G. Survival of bacteria in seawater using a diffusion chamber apparatus in situ. Appl Environ Microbiol. 1976 Jun;31(6):913–920. doi: 10.1128/aem.31.6.913-920.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


