Abstract
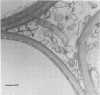
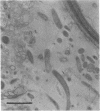
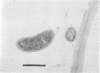
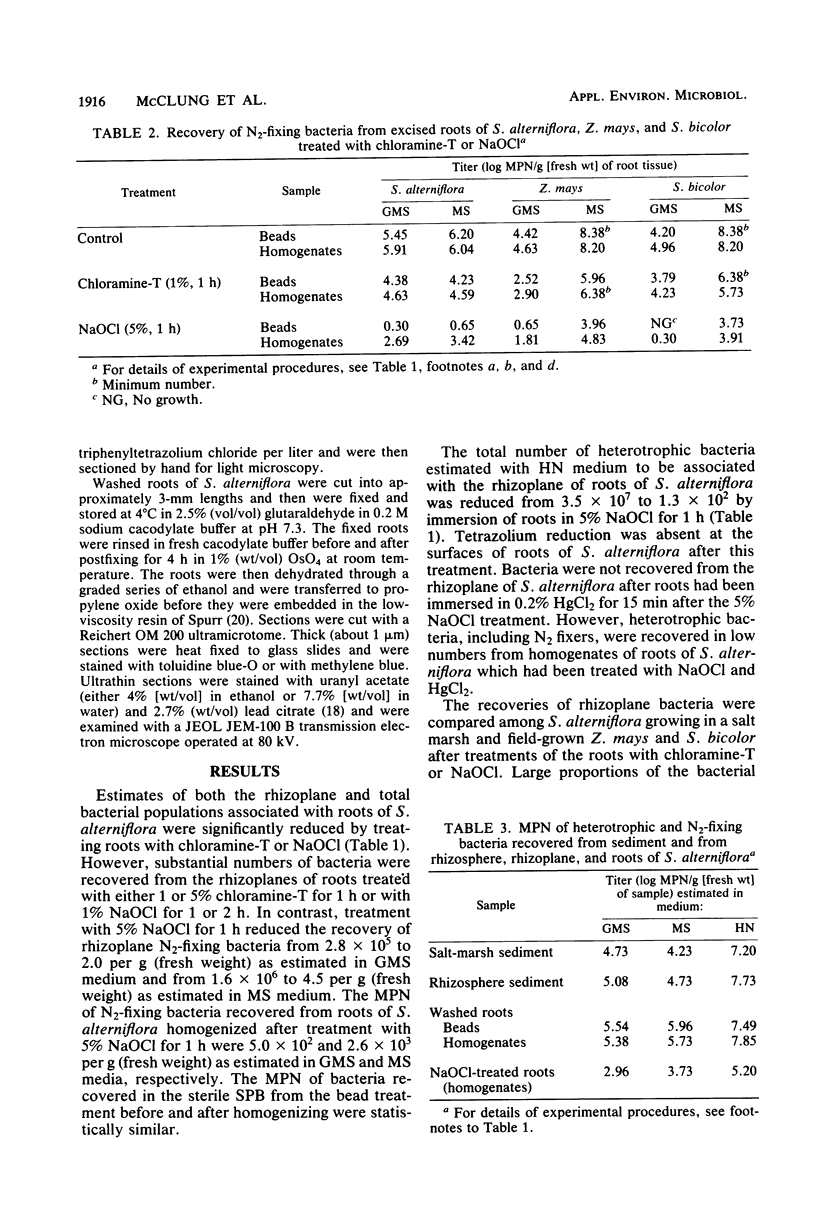
Numbers and possible locations of N2-fixing bacteria were investigated in roots of Spartina alterniflora Loisel, which support nitrogenase activity in the undisturbed native habitat. N2-fixing bacteria were recovered in cultures both from S. alterniflora roots and from the surrounding sediment, and they formed a greater proportion of the bacteria recovered from root homogenates than from salt-marsh sediment. N2-fixing bacteria were recovered in high numbers from the rhizoplane of S. alterniflora after roots were treated with 1 or 5% chloramine-T for 1 h or with 1% NaOCl for 1 or 2 h. Immersing S. alterniflora roots in 5% NaOCl for 1 h was more effective in distinguishing bacteria inside the roots since this treatment nearly eliminated N2-fixing bacteria recoverable from the rhizoplane, although high numbers of N2-fixing bacteria were recovered from homogenates of roots treated with 5% NaOCl for 1 h. However, this treatment was less effective with roots of Zea mays L. (Funks G4646) and Sorghum bicolor (L.) Moench (CK-60 A), indicating that techniques to surface sterilize roots should be evaluated for different plants. Bacteria were observed by light and electron microscopy inter- and intracellularly in the cortex and in the aerenchyma of S. alterniflora roots. This study clearly shows that bacteria, including N2 fixers, colonize the interior of roots of S. alterniflora growing in a Chesapeake Bay, Maryland, salt marsh.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle C. D., Patriquin D. G. Endorhizal and Exorhizal Acetylene-reducing Activity in a Grass (Spartina alterniflora Loisel.)-Diazotroph Association. Plant Physiol. 1980 Aug;66(2):276–280. doi: 10.1104/pp.66.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Döbereiner J., Baldani V. L. Selective infection of maize roots by streptomycin-resistant Azospirillum lipoferum and other bacteria. Can J Microbiol. 1979 Nov;25(11):1264–1269. doi: 10.1139/m79-199. [DOI] [PubMed] [Google Scholar]
- McClung C. R., Patriquin D. G. Isolation of a nitrogen-fixing Campylobacter species from the roots of Spartina alterniflora Loisel. Can J Microbiol. 1980 Aug;26(8):881–886. doi: 10.1139/m80-153. [DOI] [PubMed] [Google Scholar]
- Patriquin D. G., Döbereiner J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can J Microbiol. 1978 Jun;24(6):734–742. doi: 10.1139/m78-122. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Barraquio W. L., De Guzman M. R., Cabrera D. A. Nitrogen-fixing (acetylene redution) activity and population of aerobic heterotrophic nitrogen-fixing bacteria associated with wetland rice. Appl Environ Microbiol. 1979 May;37(5):813–819. doi: 10.1128/aem.37.5.813-819.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P., Bohlool B. B. Evaluation of nitrogen fixation by bacteria in association with roots of tropical grasses. Microbiol Rev. 1980 Sep;44(3):491–517. doi: 10.1128/mr.44.3.491-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P., Sloger C. Comparing time course profiles of immediate acetylene reduction by grasses and legumes. Appl Environ Microbiol. 1981 Jan;41(1):184–189. doi: 10.1128/aem.41.1.184-189.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P., Sloger C. Immediate acetylene reduction by excised grass roots not previously preincubated at low oxygen tensions. Plant Physiol. 1979 Nov;64(5):739–743. doi: 10.1104/pp.64.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]