Abstract
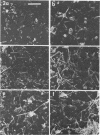
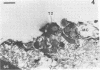
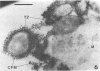
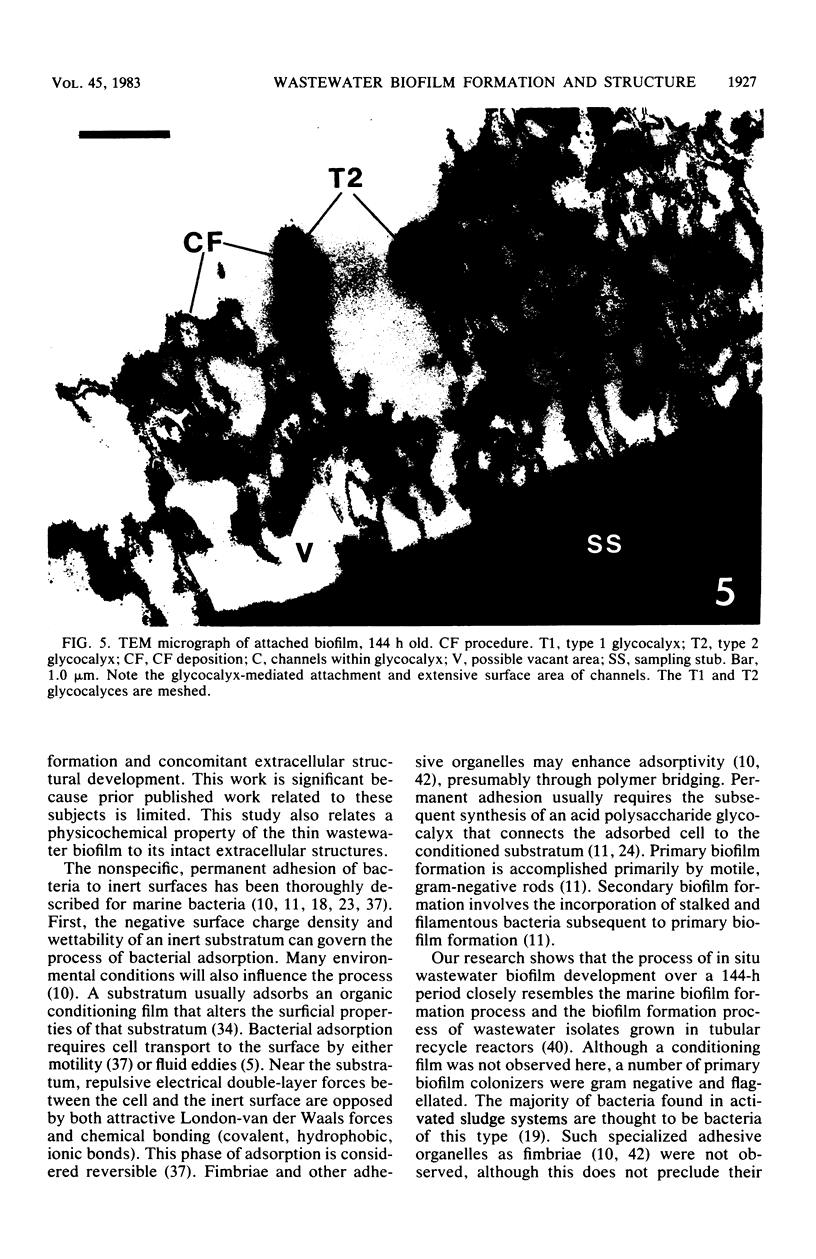
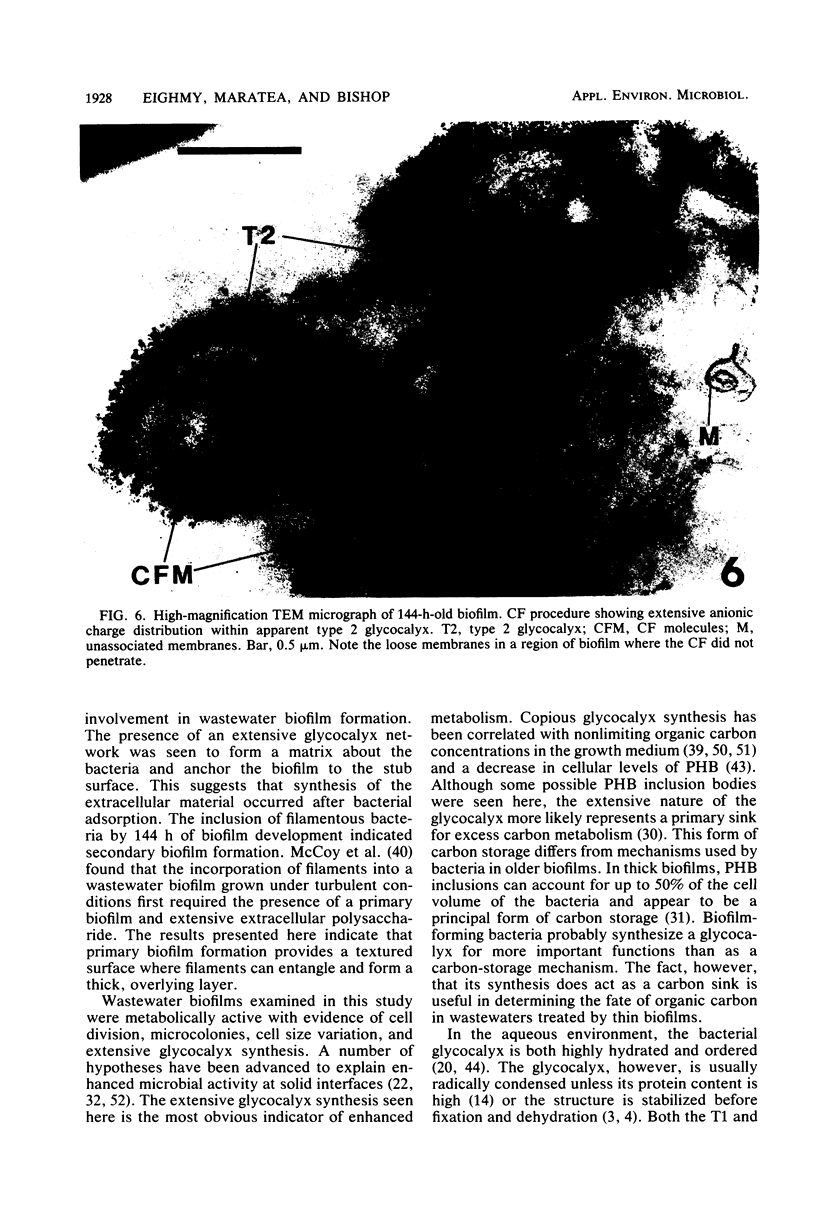
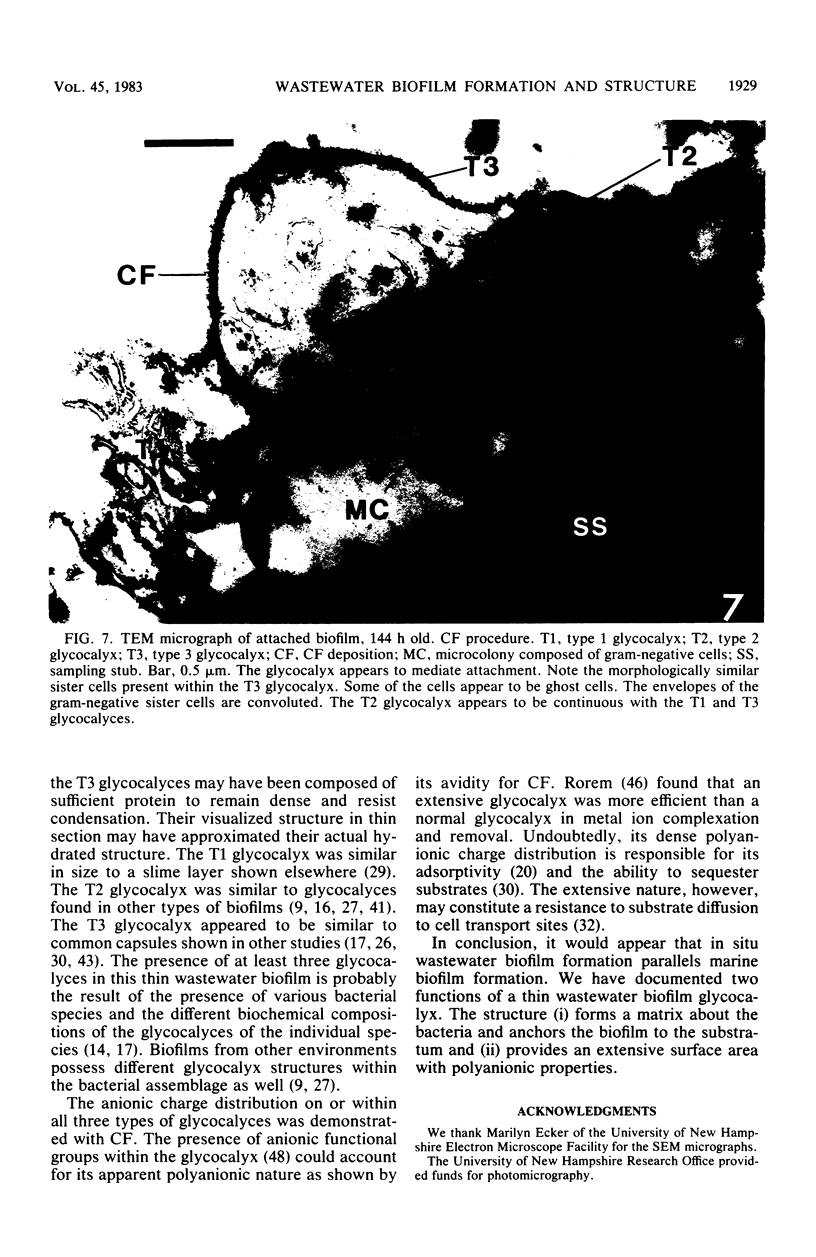
This research documents in situ wastewater biofilm formation, structure, and physiochemical properties as revealed by scanning and transmission electron microscopy. Cationized ferritin was used to label anionic sites of the biofilm glycocalyx for viewing in thin section. Wastewater biofilm formation paralleled the processes involved in marine biofilm formation. Scanning electron microscopy revealed a dramatic increase in cell colonization and growth over a 144-h period. Constituents included a variety of actively dividing morphological types. Many of the colonizing bacteria were flagellated. Filaments were seen after primary colonization of the surface. Transmission electron microscopy revealed a dominant gram-negative cell wall structure in the biofilm constituents. At least three types of glycocalyces were observed. The predominant glycocalyx possessed interstices and was densely labeled with cationized ferritin. Two of the glycocalyces appeared to mediate biofilm adhesion to the substratum. The results suggest that the predominant glycocalyx of this thin wastewater biofilm serves, in part, to: (i) enclose the bacteria in a matrix and anchor the biofilm to the substratum and (ii) provide an extensive surface area with polyanionic properties.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill D. L., Casida L. E. Attachment to autoclaved soil of bacterial cells from pure cultures of soil isolates. Appl Environ Microbiol. 1979 May;37(5):1031–1037. doi: 10.1128/aem.37.5.1031-1037.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D., Pfister R. M., Vela G. R. Improved staining of extracellular polymer for electron microscopy: examination of Azotobacter, Zoogloea, Leuconostoc, and Bacillus. Appl Microbiol. 1972 Sep;24(3):477–487. doi: 10.1128/am.24.3.477-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. The formation of microcolonies by rumen bacteria. Can J Microbiol. 1980 Sep;26(9):1104–1113. doi: 10.1139/m80-183. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Irvin R. T., Costerton J. W. Autochthonous and pathogenic colonization of animal tissues by bacteria. Can J Microbiol. 1981 May;27(5):461–490. doi: 10.1139/m81-071. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- DIAS F. F., BHAT J. V. MICROBIAL ECOLOGY OF ACTIVATED SLUDGE. I. DOMINANT BACTERIA. Appl Microbiol. 1964 Sep;12:412–417. doi: 10.1128/am.12.5.412-417.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Dunlop W. F., Robards A. W. Ultrastructural study of poly- -hydroxybutyrate granules from Bacillus cereus. J Bacteriol. 1973 Jun;114(3):1271–1280. doi: 10.1128/jb.114.3.1271-1280.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Keevil C. W., Marsh P. D., Brown C. M., Wardell J. N. Surface-associated growth. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):517–532. doi: 10.1098/rstb.1982.0058. [DOI] [PubMed] [Google Scholar]
- Fletcher M., Loeb G. I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol. 1979 Jan;37(1):67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R., Pfister R. M., Remsen C. C. Fine structure and composition of the zoogloeal matrix surrounding Zoogloea ramigera. J Bacteriol. 1968 Dec;96(6):2144–2153. doi: 10.1128/jb.96.6.2144-2153.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geesey G. G., Richardson W. T., Yeomans H. G., Irvin R. T., Costerton J. W. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can J Microbiol. 1977 Dec;23(12):1733–1736. doi: 10.1139/m77-249. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W., Whiteside T. L. Mesosomes: membranous bacterial organelles. Bacteriol Rev. 1975 Dec;39(4):405–463. doi: 10.1128/br.39.4.405-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner N. E., Balkwill D. L., Bishop P. L. Light and electron microscopic studies of microorganisms growing in rotating biological contactor biofilms. Appl Environ Microbiol. 1983 May;45(5):1659–1669. doi: 10.1128/aem.45.5.1659-1669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Motta E. J. Kinetics of growth and substrate uptake in a biological film system. Appl Environ Microbiol. 1976 Feb;31(2):286–293. doi: 10.1128/aem.31.2.286-293.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- MacaAlister T. J., Irvin R. T., Costerton J. W. Cell surface-localized alkaline phosphatase of Escherichia coli as visualized by reaction product deposition and ferritin-labeled antibodies. J Bacteriol. 1977 Apr;130(1):318–328. doi: 10.1128/jb.130.1.318-328.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall K. C., Stout R., Mitchell R. Selective sorption of bacteria from seawater. Can J Microbiol. 1971 Nov;17(11):1413–1416. doi: 10.1139/m71-225. [DOI] [PubMed] [Google Scholar]
- McCoy W. F., Bryers J. D., Robbins J., Costerton J. W. Observations of fouling biofilm formation. Can J Microbiol. 1981 Sep;27(9):910–917. doi: 10.1139/m81-143. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Parsons A. B., Dugan P. R. Production of extracellular polysaccharide matrix by Zoogloea ramigera. Appl Microbiol. 1971 Apr;21(4):657–661. doi: 10.1128/am.21.4.657-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROREM E. S. Uptake of rubidium and phosphate ions by polysaccharide-producing bacteria. J Bacteriol. 1955 Dec;70(6):691–701. doi: 10.1128/jb.70.6.691-701.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Wimpenny J. W. Exopolysaccharide production by Pseudomonas NCIB11264 grown in continuous culture. J Gen Microbiol. 1978 Jan;104(1):47–57. doi: 10.1099/00221287-104-1-47. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Cellular glycogen, beta-1,2,-glucan, poly beta-hydroxybutyric acid and extracellular polysaccharides in fast-growing species of Rhizobium. Antonie Van Leeuwenhoek. 1981;47(6):481–497. doi: 10.1007/BF00443236. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]