Abstract
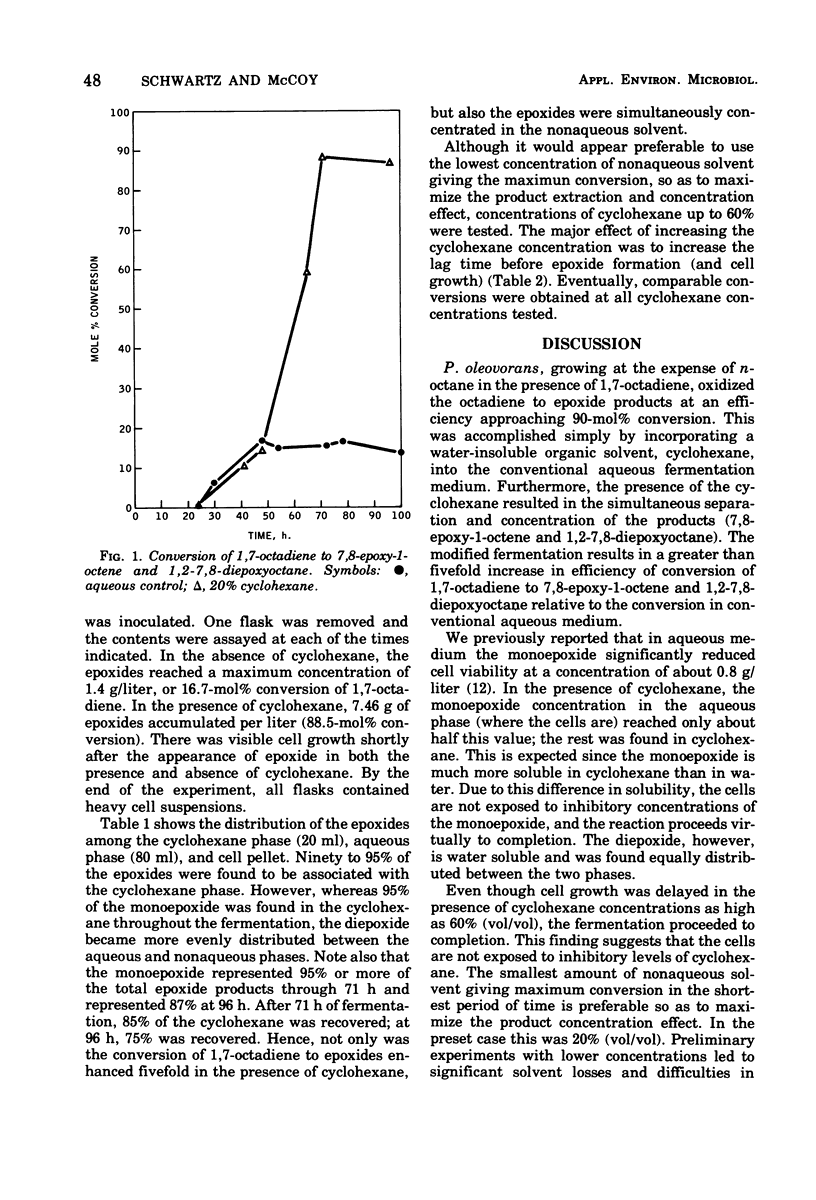
A very efficient conversion of 1,7-octadiene to 7,8-epoxy-1-octene and 1,2-7,8-diepoxyoctane was achieved by incorporating a high concentration of cyclohexane into the conventional fermentation medium. In the presence of cyclohexane, a 90-ml% conversion of substrate to product was accomplished within 72 h, compared with an 18,5-mol% conversion in the absence of cyclohexane. Furthermore, the products were simultaneously separated and concentrated in the organic phase.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Hou C. T. Oxidation of 1-alkenes to 1,2-epoxyalkanes by Pseudomonas oleovorans. Appl Microbiol. 1973 Jul;26(1):86–91. doi: 10.1128/am.26.1.86-91.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. K., Perlman D. Stimulation by organic solvents and detergents of conversion of L-sorbose to L-sorbosone by Gluconobacter melanogenus IFO 3293. Biotechnol Bioeng. 1975 Oct;17(10):1473–1483. doi: 10.1002/bit.260171008. [DOI] [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. I. Alkene epoxidation by the -hydroxylation system of Pseudomonas oleovorans. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1230–1234. doi: 10.1016/0006-291x(72)90842-x. [DOI] [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. II. Comparison between the epoxidation and hydroxylation reactions catalyzed by the -hydroxylation system of Pseudomonas oleovorans. J Biol Chem. 1973 Mar 10;248(5):1725–1730. [PubMed] [Google Scholar]
- May S. W., Abbott B. J., Felix A. On the role of superoxide in reactions catalyzed by rubredoxin of Pseudomonas oleovorans. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1540–1545. doi: 10.1016/0006-291x(73)91161-3. [DOI] [PubMed] [Google Scholar]
- May S. W., Schwartz R. D., Abbott B. J., Zaborsky O. R. Structural effects on the reactivity of substrates and inhibitors in the epoxidation system of Pseudomonas oleovorans. Biochim Biophys Acta. 1975 Sep 22;403(1):245–255. doi: 10.1016/0005-2744(75)90026-1. [DOI] [PubMed] [Google Scholar]
- May S. W., Schwartz R. D. Stereoselective epoxidation of octadiene catalyzed by an enzyme system of Pseudomonas oleovorans. J Am Chem Soc. 1974 Jun 12;96(12):4031–4032. doi: 10.1021/ja00819a060. [DOI] [PubMed] [Google Scholar]
- Schwartz R. D., McCoy C. J. Enzymatic epoxidation: synthesis of 7,8-epoxy-1-octene, 1,2-7,8-diepoxyoctane, and 1,2-Epoxyoctane by Pseudomonas oleovorans. Appl Environ Microbiol. 1976 Jan;31(1):78–82. doi: 10.1128/aem.31.1.78-82.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D., McCoy C. J. Pseudomonas oleovorans hydroxylation-epoxidation system: additional strain improvements. Appl Microbiol. 1973 Aug;26(2):217–218. doi: 10.1128/am.26.2.217-218.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D. Octene epoxidation by a cold-stable alkane-oxidizing isolate of Pseudomonas oleovorans. Appl Microbiol. 1973 Apr;25(4):574–577. doi: 10.1128/am.25.4.574-577.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Horvath C. Behavior of soluble and immobilized acid phosphatase in hydro-organic media. Biochim Biophys Acta. 1975 Nov 20;410(1):135–144. doi: 10.1016/0005-2744(75)90214-4. [DOI] [PubMed] [Google Scholar]
- Weetall H. H., Vann W. P. Studies on immobilized trypsin in high concentrations of organic solvents. Biotechnol Bioeng. 1976 Jan;18(1):105–118. doi: 10.1002/bit.260180109. [DOI] [PubMed] [Google Scholar]


