Abstract
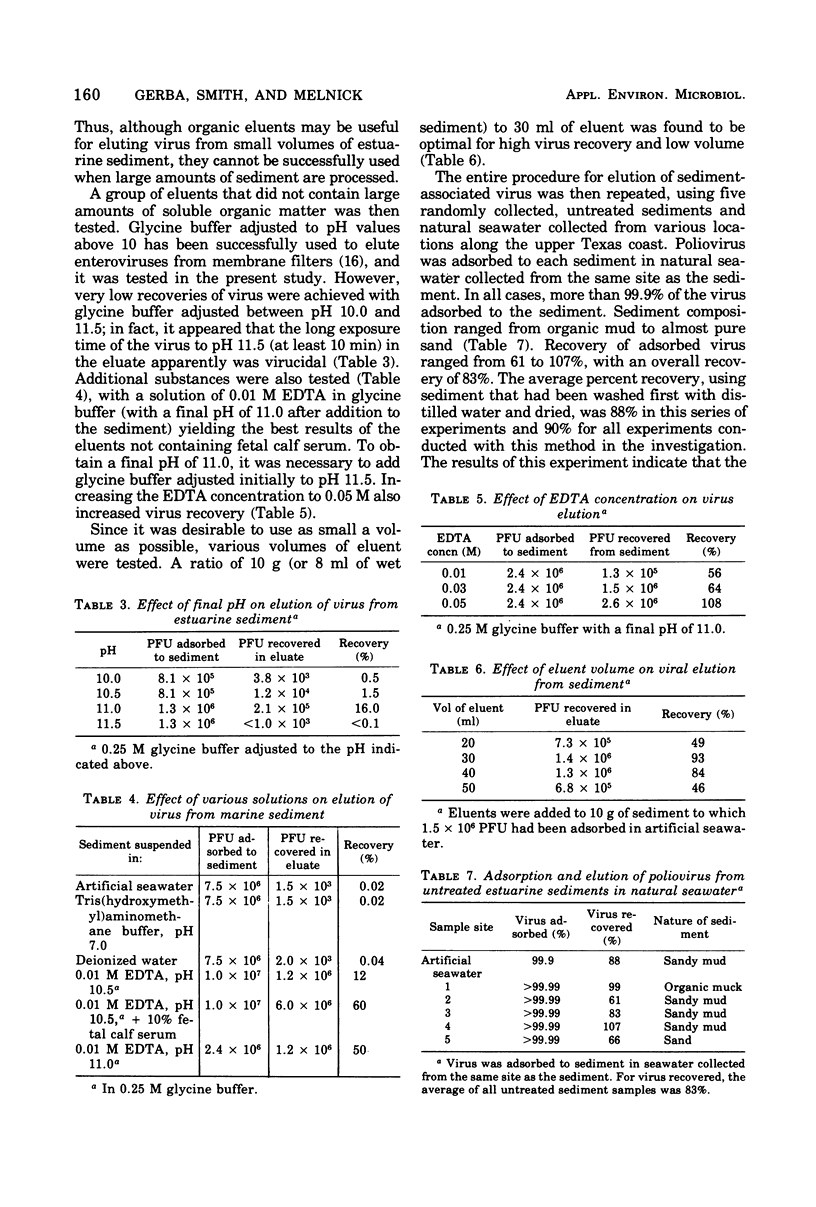
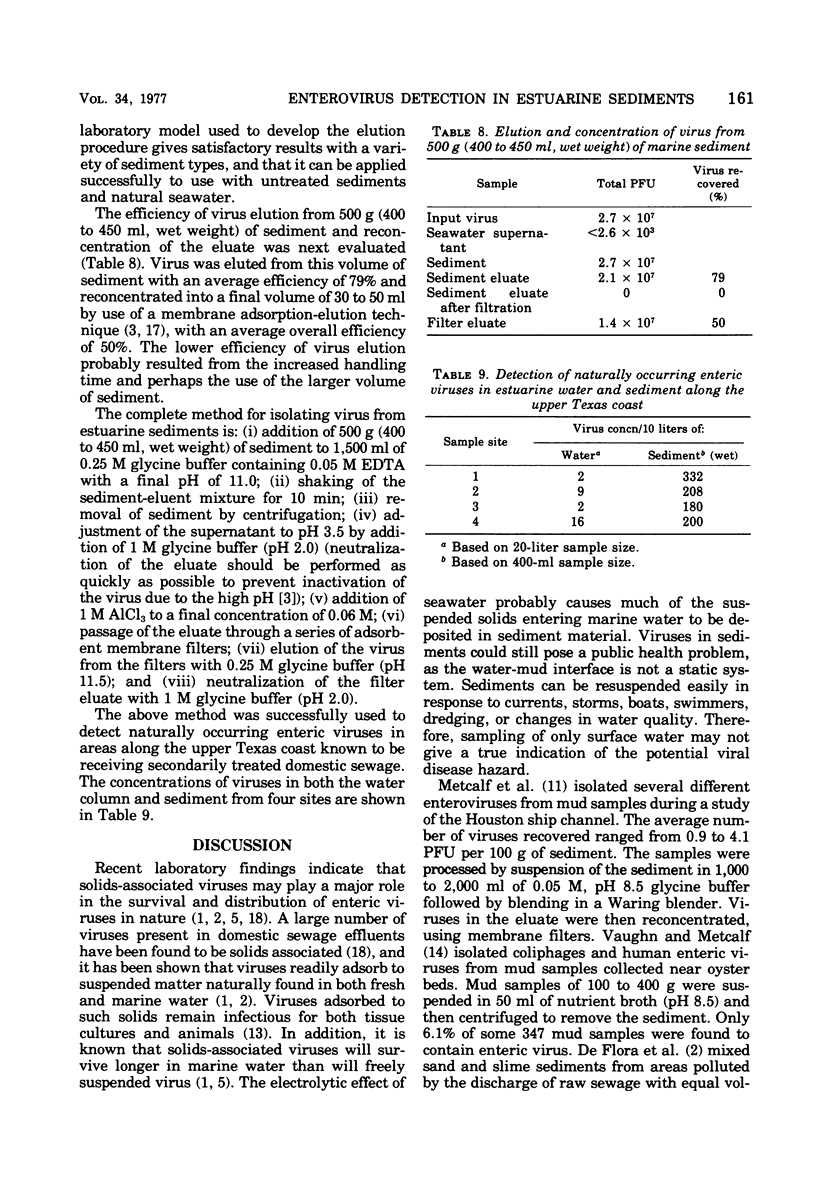
Several investigators have reported on the detection of enteric viruses in marine sediments, but none determined the efficiency of their methods and only limited volumes of sediment were sampled. The purpose of this investigation was to develop a quantitative method for detecting enteroviruses in marine sediments so that their relative proportion to viruses freely suspended in estuarine water could be more accurately determined. Poliovirus was found to adsorb readily to natural marine sediments collected along the Texas Gulf coast. A number of substances were evaluated for their ability to elute adsorbed viruses. A solution of 10% fetal calf serum adjusted to pH 10.5 and 0.05M ethylenediaminetetraacetate (pH 11.0) were found to be the best eluents. Using ethylenediaminetetraacetate as an eluent, it was possible to elute virus from large volumes of sediment and reconcentrate the sediment eluate into an economically assayable volume (30 to 50 ml). Poliovirus could be recovered from the sediment with an overall efficiency of 50%. This method was found to be satisfactory for the recovery of naturally occurring animal viruses in estuarine sediments from the upper Texas Gulf coast.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Flora S., De Renzi G. P., Badolati G. Detection of animal viruses in coastal seawater and sediments. Appl Microbiol. 1975 Sep;30(3):472–475. doi: 10.1128/am.30.3.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., McLeod J. S. Effect of sediments on the survival of Escherichia coli in marine waters. Appl Environ Microbiol. 1976 Jul;32(1):114–120. doi: 10.1128/aem.32.1.114-120.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Schaiberger G. E. Effect of particulates on virus survival in seawater. J Water Pollut Control Fed. 1975 Jan;47(1):93–103. [PubMed] [Google Scholar]
- Gerba C. P., Wallis C., Melnick J. L. Microbiological hazards of household toilets: droplet production and the fate of residual organisms. Appl Microbiol. 1975 Aug;30(2):229–237. doi: 10.1128/am.30.2.229-237.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak P. A., Caraway C. T., Portnoy B. L. Oyster-associated hepatitis: lessons from the Louisiana experience. Am J Epidemiol. 1976 Feb;103(2):181–191. doi: 10.1093/oxfordjournals.aje.a112216. [DOI] [PubMed] [Google Scholar]
- Matossian A. M., Garabedian G. A. Virucidal action of sea water. Am J Epidemiol. 1967 Jan;85(1):1–8. doi: 10.1093/oxfordjournals.aje.a120666. [DOI] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J. L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972 Mar;23(3):476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


