Abstract
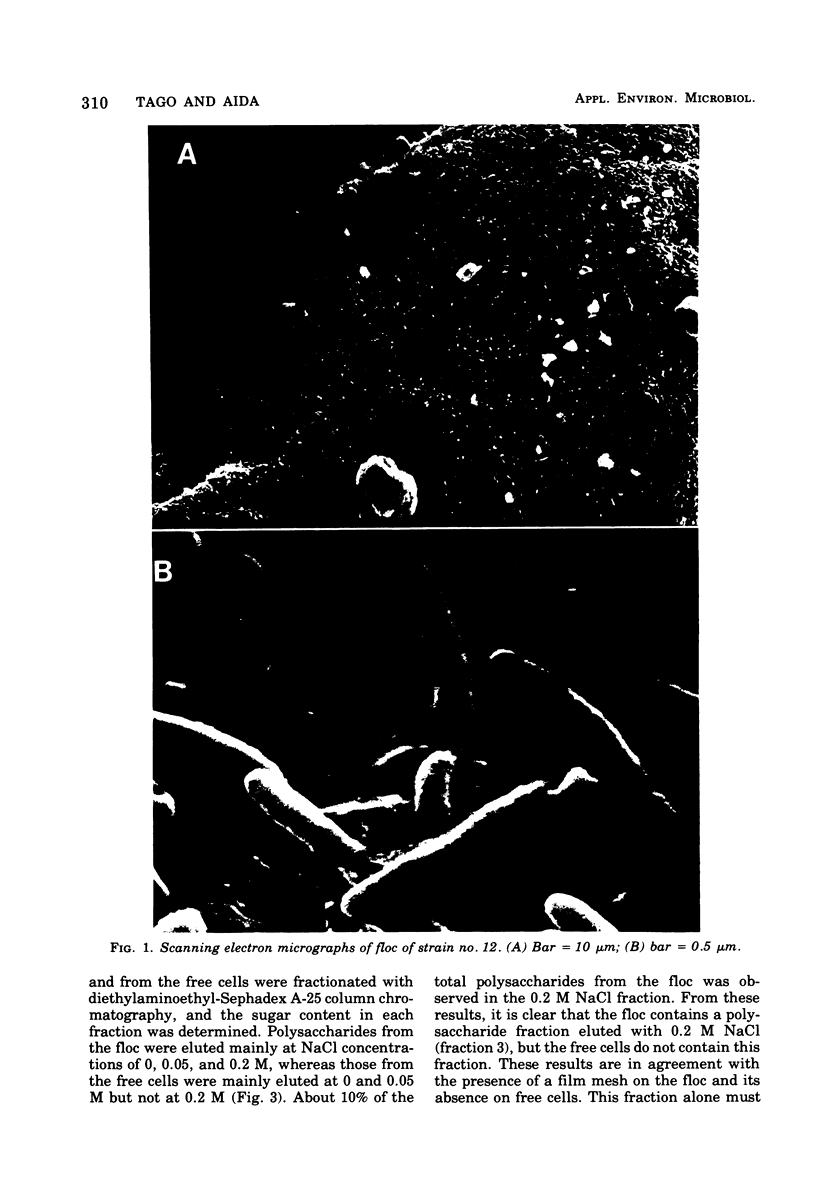
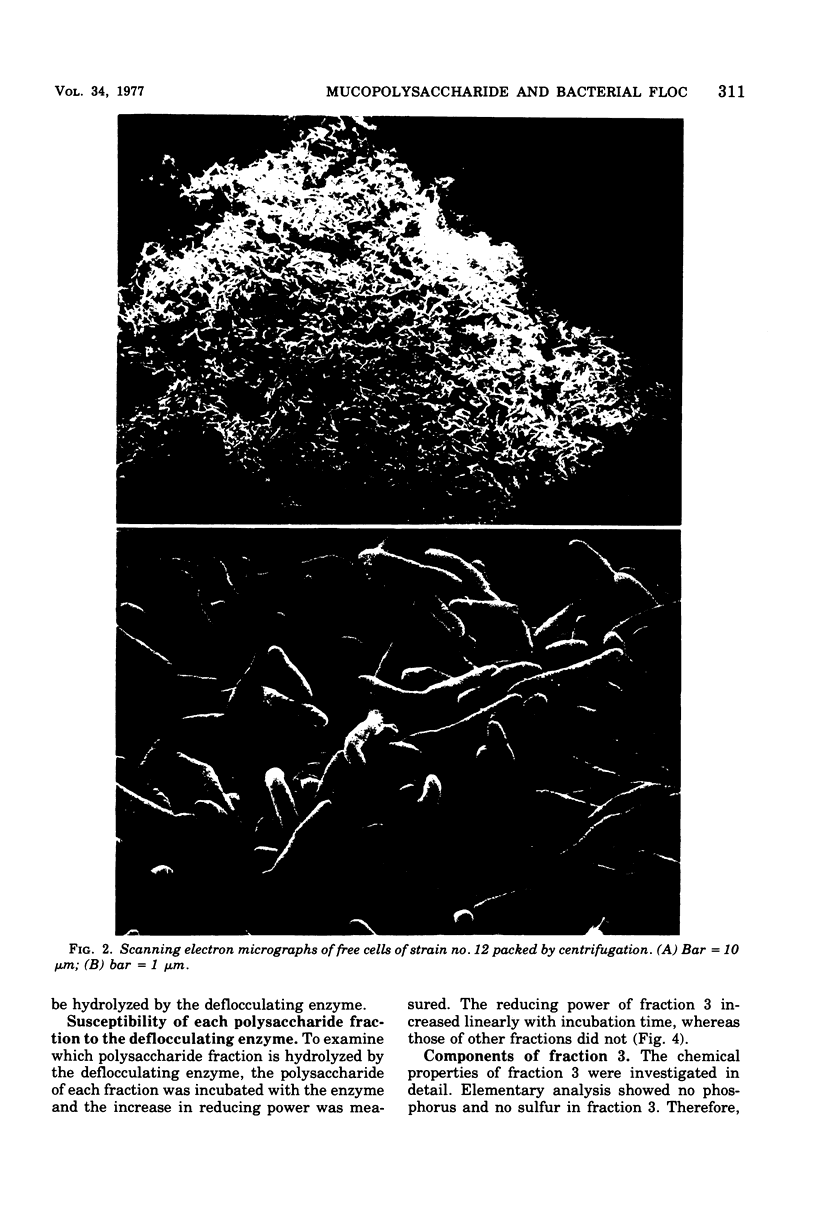
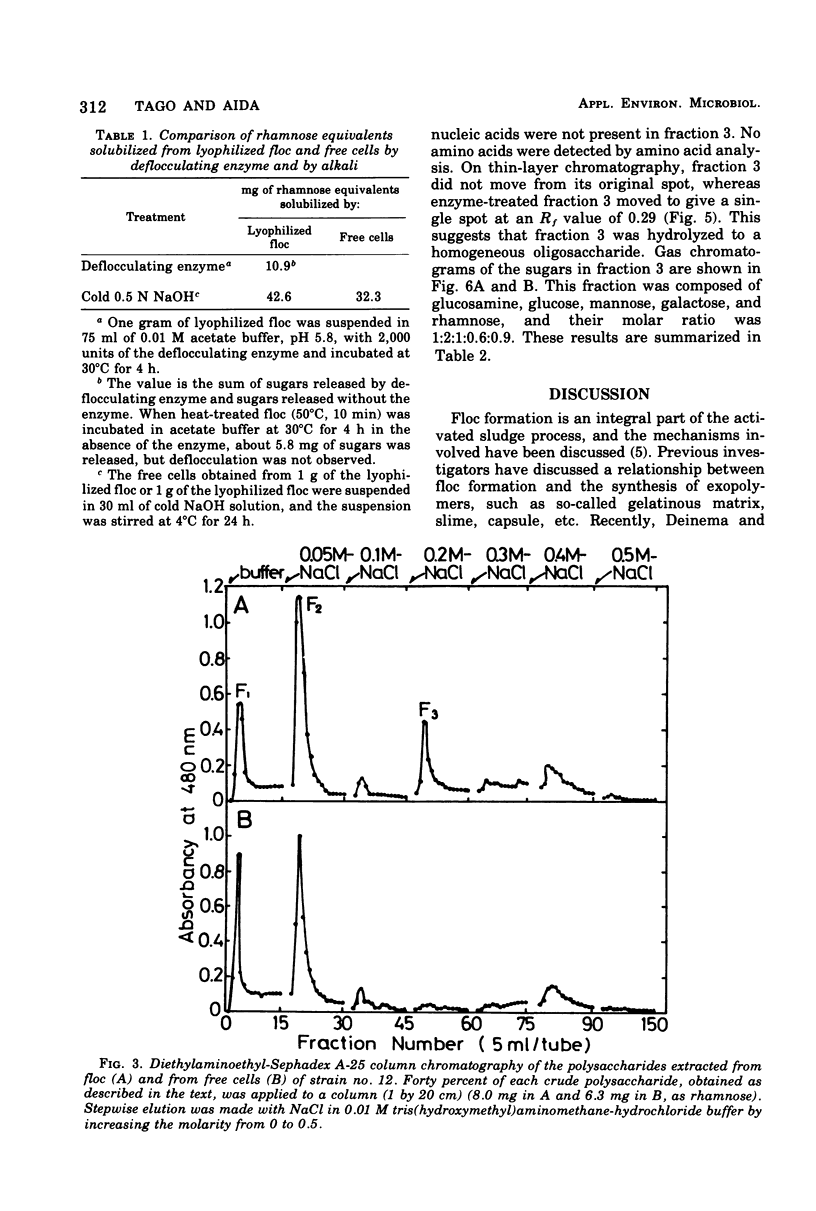
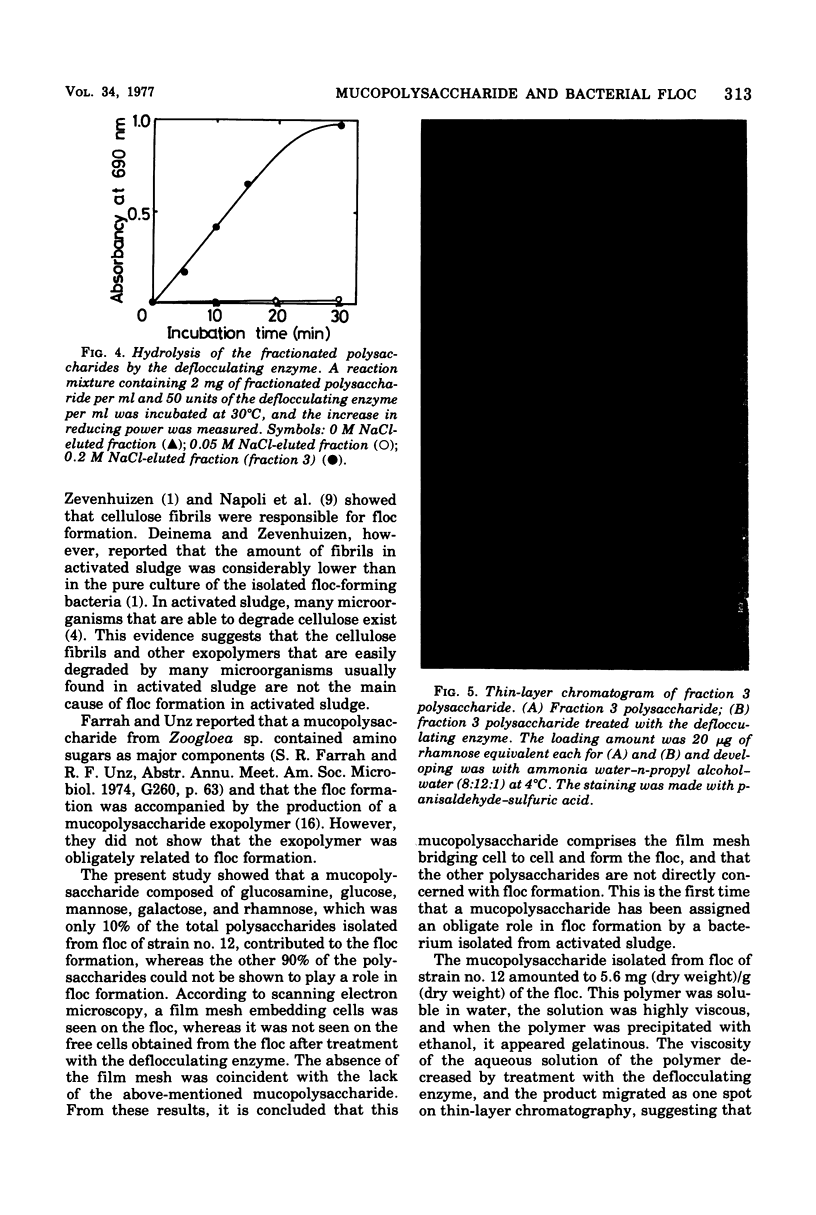
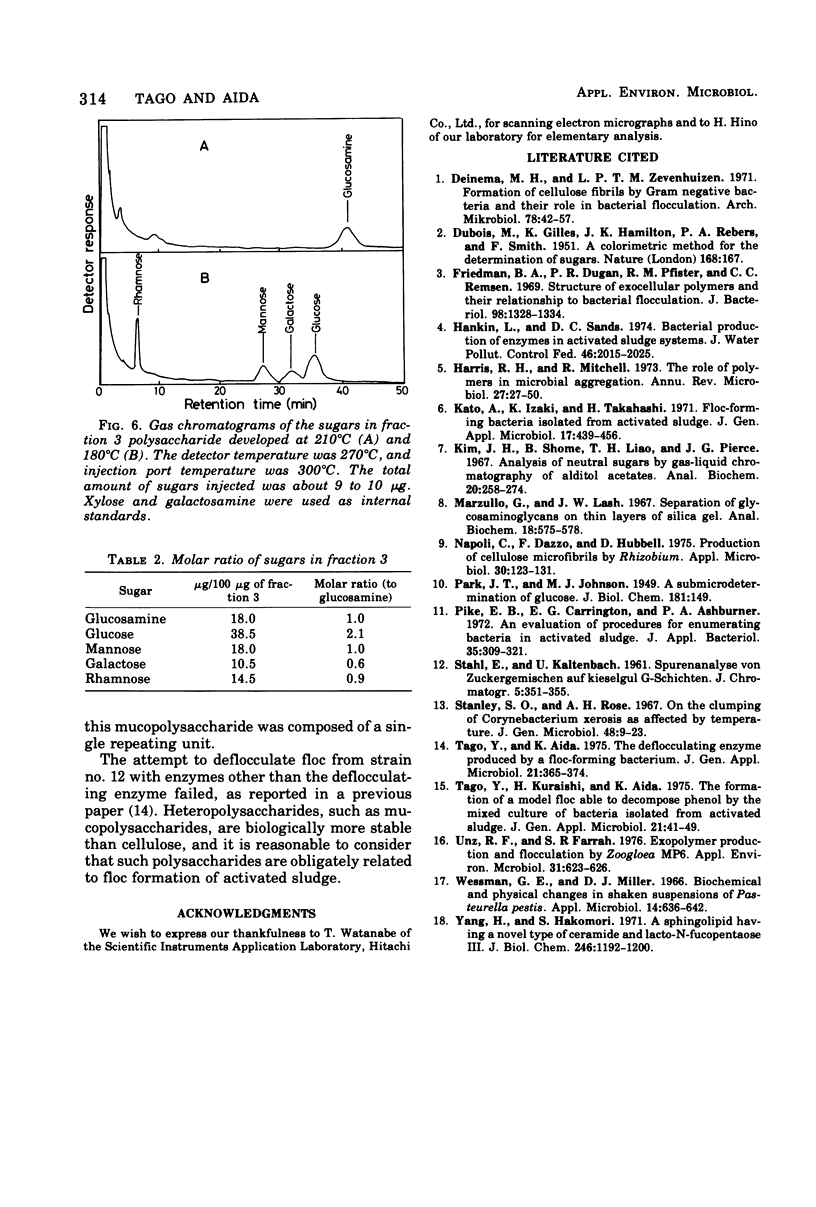
A bacterium isolated from activated sludge formed a visible floc and also produced an exoenzyme that could bring about deflocculation. Scanning electron microscopic examination revealed that the cells were embedded in a film mesh in the floc, which disappeared after treatment with the deflocculating enzyme. Polysaccharides isolated from the floc were fractionated into three fractions by diethylaminoethyl-Sephadex A-25 column chromatography, whereas those from the free cells were fractionated into only two fractions. The missing fraction was a mucopolysaccharide composed of glucosamine, glucose, mannose, galactose, and rhamnose and was hydrolyzed to oligosaccharides by the deflocculating enzyme. The other two fractions were resistant to the enzyme. These results show that the mesh structure of the floc is dependent on a mucopolysaccharide hydrolyzed by the deflocculating enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBOIS M., GILLES K., HAMILTON J. K., REBERS P. A., SMITH F. A colorimetric method for the determination of sugars. Nature. 1951 Jul 28;168(4265):167–167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R., Pfister R. M., Remsen C. C. Structure of exocellular polymers and their relationship to bacterial flocculation. J Bacteriol. 1969 Jun;98(3):1328–1334. doi: 10.1128/jb.98.3.1328-1334.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. H., Mitchell R. The role of polymers in microbial aggregation. Annu Rev Microbiol. 1973;27:27–50. doi: 10.1146/annurev.mi.27.100173.000331. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Shome B., Liao T. H., Pierce J. G. Analysis of neutral sugars by gas-liquid chromatography of alditol acetates: application to thyrotropic hormone and other glycoproteins. Anal Biochem. 1967 Aug;20(2):258–274. doi: 10.1016/0003-2697(67)90031-0. [DOI] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Pike E. B., Carrington E. G., Ashburner P. A. An evaluation of procedures for enumerating bacteria in activated sludge. J Appl Bacteriol. 1972 Jun;35(2):309–321. doi: 10.1111/j.1365-2672.1972.tb03703.x. [DOI] [PubMed] [Google Scholar]
- Unz R. F., Farrah S. R. Exopolymer production and flocculation by zoogloea mp6. Appl Environ Microbiol. 1976 Apr;31(4):623–626. doi: 10.1128/aem.31.4.623-626.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessman G. E., Miller D. J. Biochemical and physical changes in shaken suspensions of Pasteurella pestis. Appl Microbiol. 1966 Jul;14(4):636–642. doi: 10.21236/ad0481042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. J., Hakomori S. I. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971 Mar 10;246(5):1192–1200. [PubMed] [Google Scholar]