Abstract
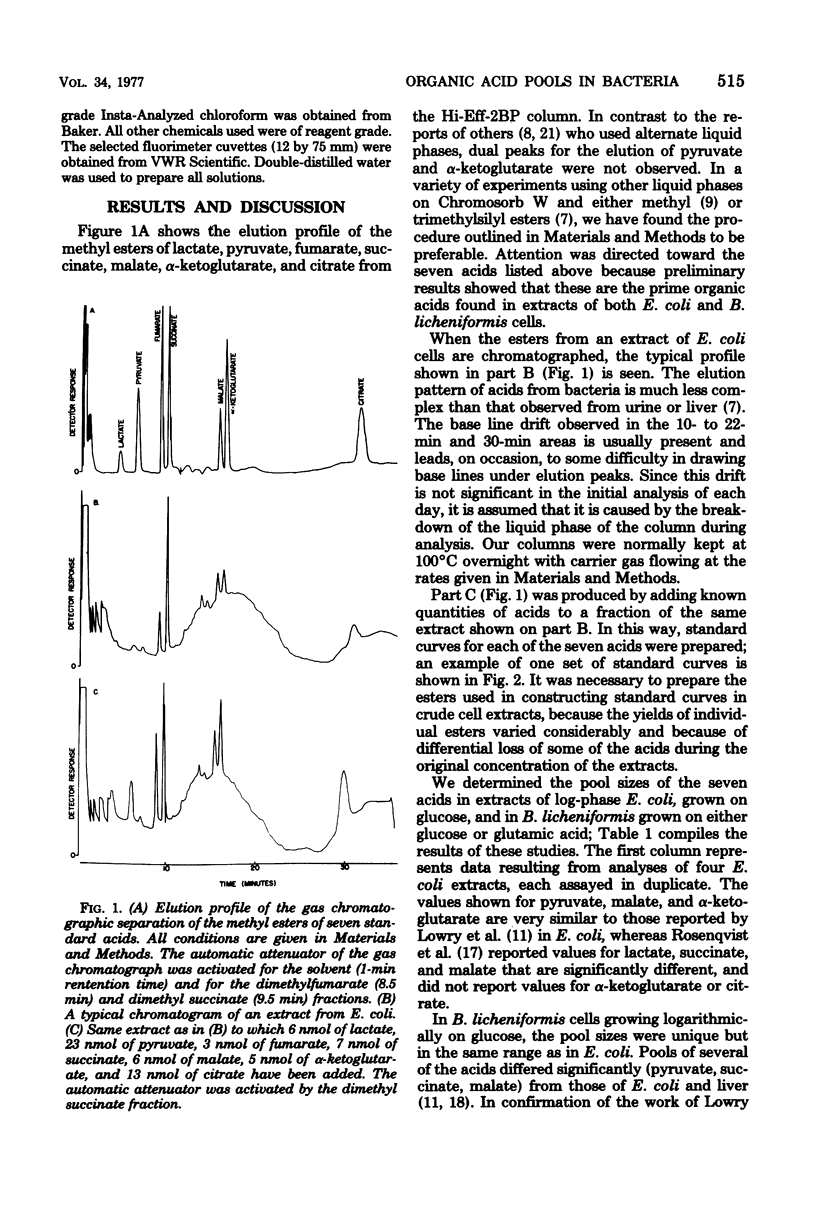
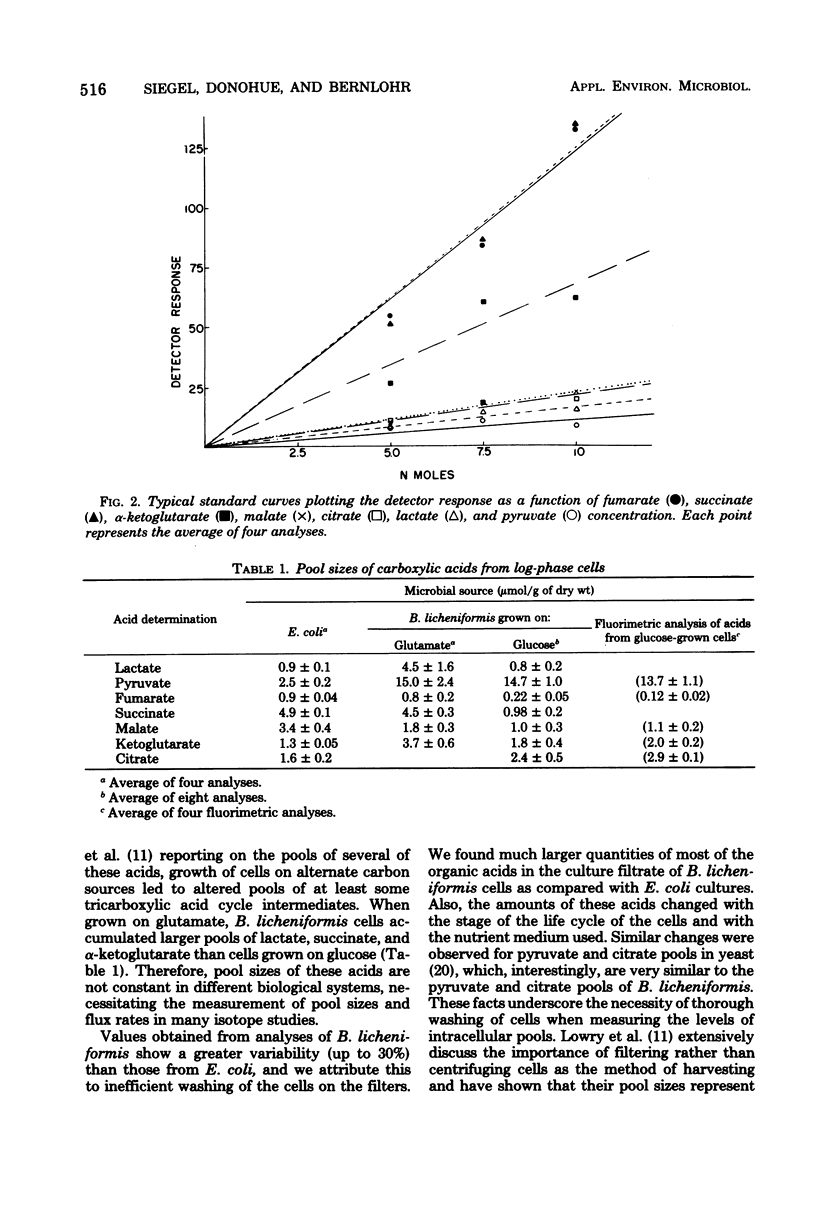
Methods for sampling, extracting, and quantitating the metabolic pools of organic acids from bacteria have been developed. The concentration of these metabolites was determined by a new gas chromatographic method that can quantitatively determine the levels of lactate, pyruvate, fumarate, succinate, malate, alpha-ketoglutarate, and citrate. Values obtained were confirmed by fluorimetric analyses of five of the individual acids. In Escherichia coli, pools range from about 1 to 5 mumol/g of dry weight, with a variation in replicate samples of 5 to 15%. Under similar conditions, these pools in Bacillus licheniformis are in the same range, although the pyruvic acid pool is significantly larger.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson I., Norkrans B., Odham G. Determination of the Krebs cycle related keto acids of microorganisms by capillary gas chromatography on glass columns. Anal Biochem. 1973 Jun;53(2):629–638. doi: 10.1016/0003-2697(73)90115-2. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Clark V. Characterization and regulation of protease synthesis and activity in Bacillus licheniformis. J Bacteriol. 1971 Jan;105(1):276–283. doi: 10.1128/jb.105.1.276-283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Peterson D. E., Bernlohr R. W. Changes in free amino acid production and intracellular amino acid pools of Bacillus licheniformis as a function of culture age and growth media. J Bacteriol. 1972 Nov;112(2):715–725. doi: 10.1128/jb.112.2.715-725.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Urban E., Schlegel H. G. Measuring the concentrations of metabolites in bacteria. Anal Biochem. 1976 May 7;72:191–201. doi: 10.1016/0003-2697(76)90521-2. [DOI] [PubMed] [Google Scholar]
- Dalgliesh C. E., Horning E. C., Horning M. G., Knox K. L., Yarger K. A gas-liquid-chromatographic procedure for separating a wide range of metabolites occuring in urine or tissue extracts. Biochem J. 1966 Dec;101(3):792–810. doi: 10.1042/bj1010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon M. A., Doelle H. W. Gas chromatographic separation and determination of microquantities of the esters of the tricarboxylic acid cycle acids and related compounds. J Chromatogr. 1969 Jun 17;42(2):157–169. doi: 10.1016/s0021-9673(01)80611-7. [DOI] [PubMed] [Google Scholar]
- Hautala E., Weaver M. L. Separation and quantitative determination of lactic, pyruvic, fumaric, succinic, malic, and citric acids by gas chromatography. Anal Biochem. 1969 Jul;30(1):32–39. doi: 10.1016/0003-2697(69)90370-4. [DOI] [PubMed] [Google Scholar]
- Ishitoya Y., Ito C., Osawa N., Hashimoto I., Iwanaga T., Nambara T. Gas chromatographic separation of tricarboxylic acid cycle components. Clin Chim Acta. 1970 Feb;27(2):233–240. doi: 10.1016/0009-8981(70)90340-2. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Moses V., Sharp P. B. Intermediary metabolite levels in Escherichia coli. J Gen Microbiol. 1972 Jun;71(1):181–190. doi: 10.1099/00221287-71-1-181. [DOI] [PubMed] [Google Scholar]
- Rosenqvist H., Kallio H., Nurmikko V. Gas chromatographic analysis of citric acid cycle and related compounds from Escherichia coli as their trimethylsilyl derivatives. Anal Biochem. 1972 Mar;46(1):224–231. doi: 10.1016/0003-2697(72)90415-0. [DOI] [PubMed] [Google Scholar]
- Sauer F., Erfle J. D., Binns M. R. Turnover rates and intracellular pool size distribution of citrate cycle intermediates in normal, diabetic and fat-fed rats estimated by computer analysis from specific activity decay data of 14C-labeled citrate cycle acids. Eur J Biochem. 1970 Dec;17(2):350–363. doi: 10.1111/j.1432-1033.1970.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. I. Purification, stability, regulation of synthesis, and evidence for multiple molecular states. J Biol Chem. 1971 Mar 25;246(6):1733–1745. [PubMed] [Google Scholar]
- Weibel K. E., Mor J. R., Fiechter A. Rapid sampling of yeast cells and automated assays of adenylate, citrate, pyruvate and glucose-6-phosphate pools. Anal Biochem. 1974 Mar;58(1):208–216. doi: 10.1016/0003-2697(74)90459-x. [DOI] [PubMed] [Google Scholar]
- Zaura D. S., Metcoff J. Quantification of seven tricarboxylic acid cycle and related acids in human urine by gas-liquid chromatography. Anal Chem. 1969 Nov;41(13):1781–1787. doi: 10.1021/ac60282a034. [DOI] [PubMed] [Google Scholar]


