Abstract
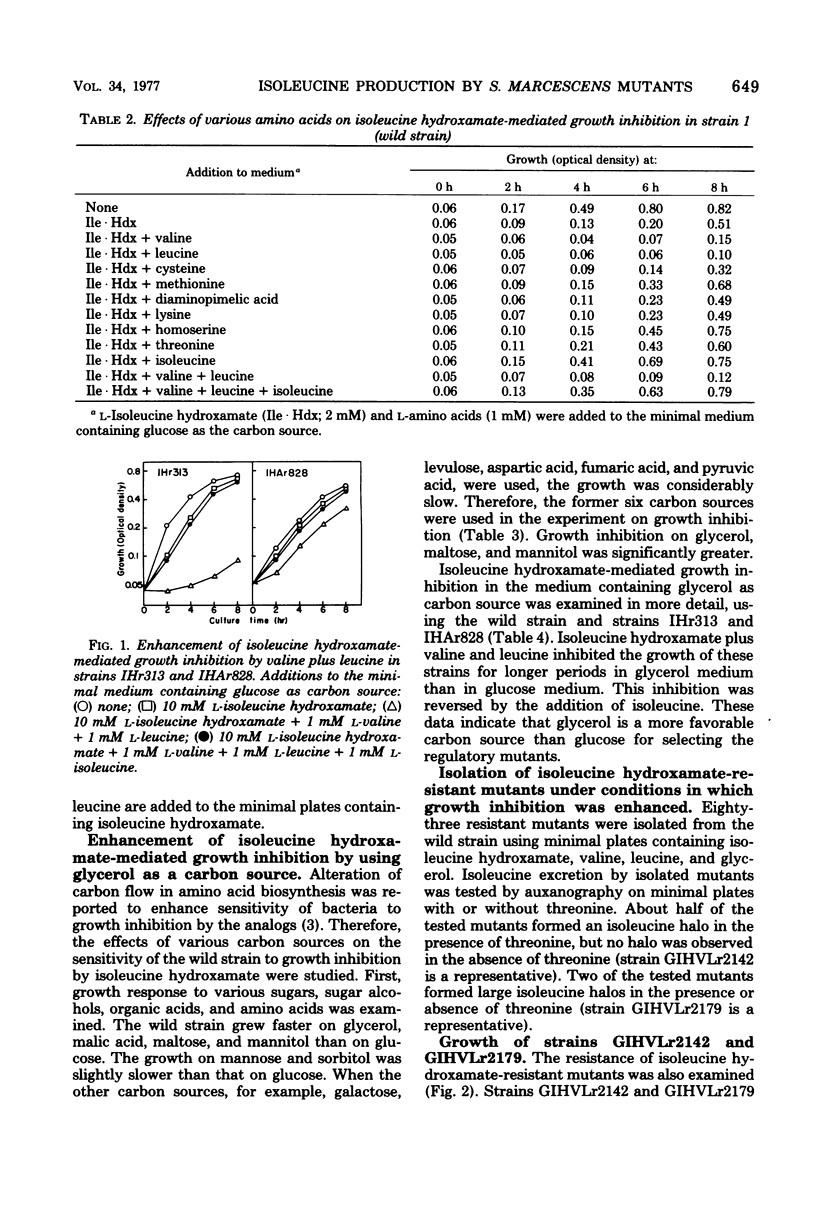
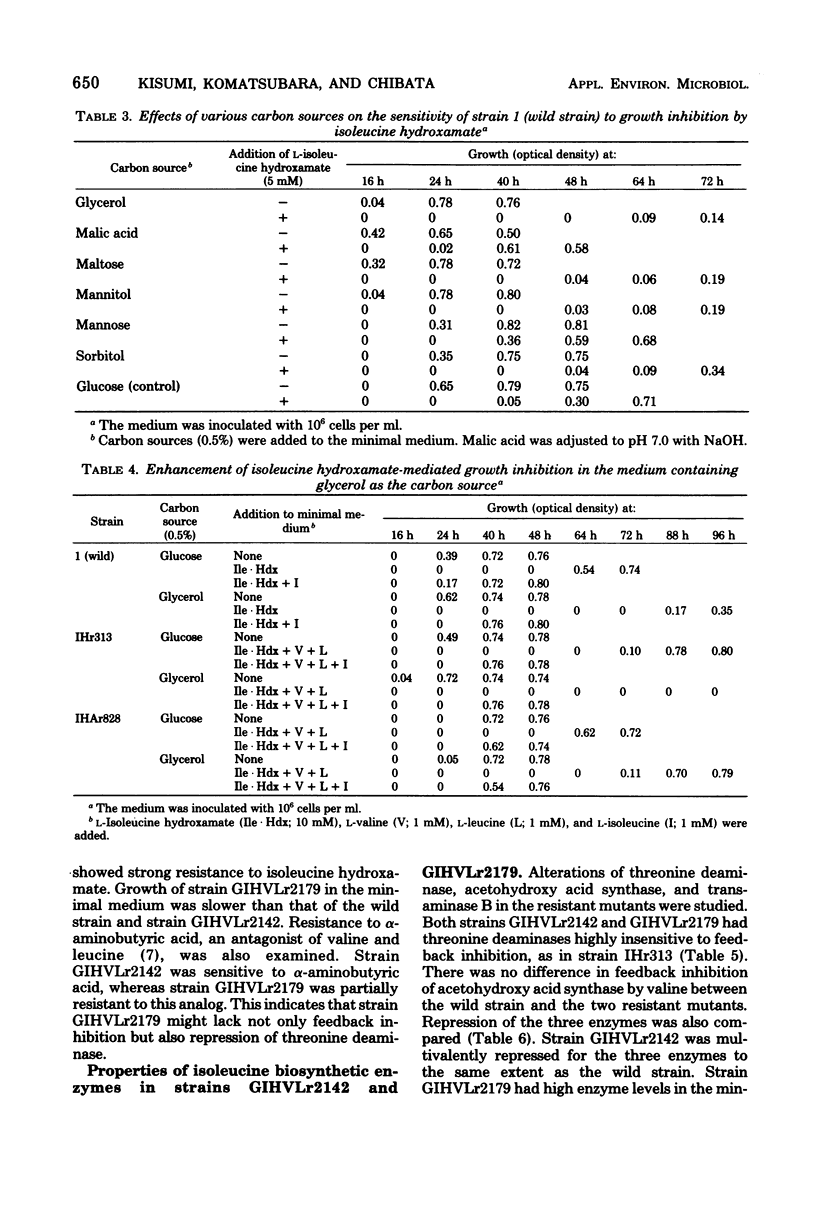
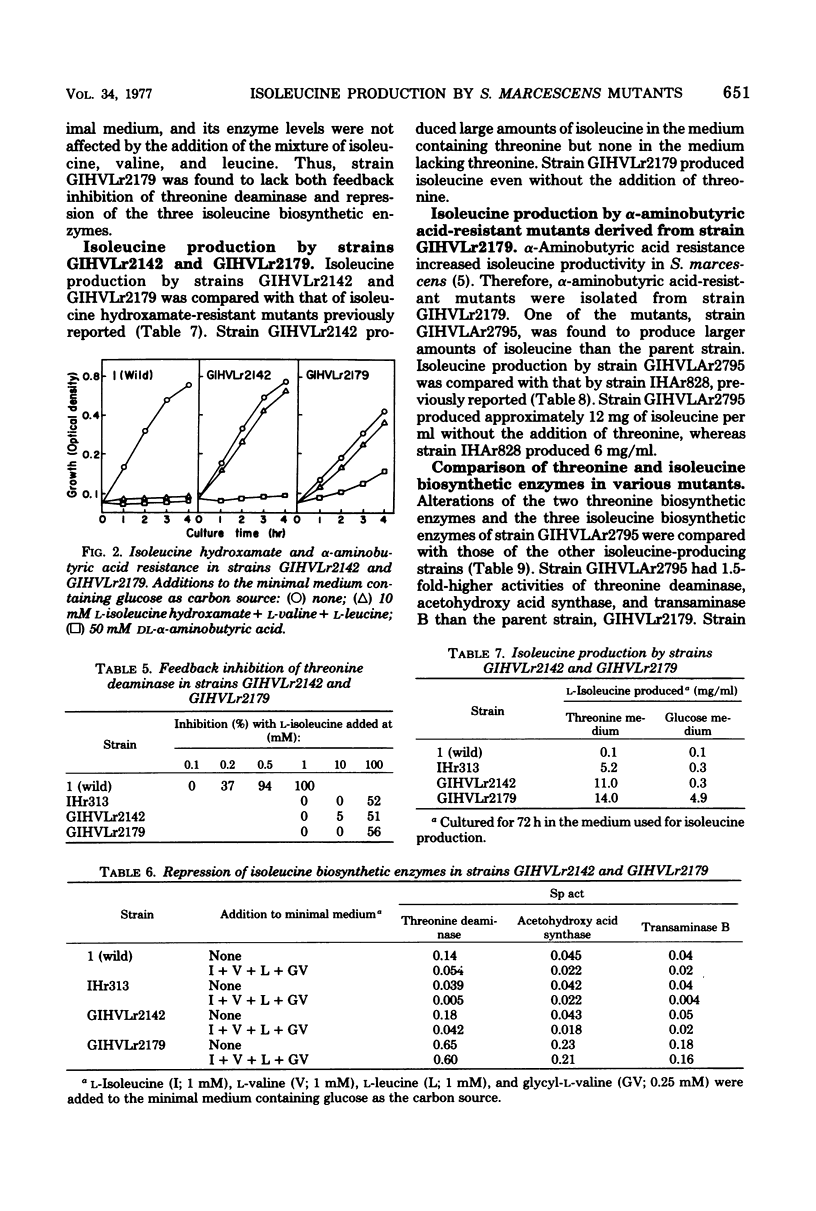
Growth inhibition by isoleucine hydroxamate in Serratia marcescens was significantly enhanced by adding valine plus leucine and by using glycerol as the carbon source. Isoleucine hydroxamate-resistant mutants were isolated under conditions in which growth inhibition was enhanced. One of the mutants, strain GIHVLr2179, lacked both feedback inhibition and repression of threonine deaminase. An alpha-aminobutyric acid-resistant mutant derived from strain GIHVLr2179, strain GIHVLAr2795, produced 12 mg of isoleucine per ml in the medium containing glucose and urea as carbon and nitrogen sources (a twofold increase over prior reports). This strain had increased activities of threonine deaminase, acetohydroxy acid synthase, aspartokinase, and homoserine dehydrogenase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A. Selection of bacterial mutants which excrete antagonists of antimetabolites. J Bacteriol. 1958 Sep;76(3):326–326. doi: 10.1128/jb.76.3.326-326.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Jensen R. A. Significance of altered carbon flow in aromatic amino acid synthesis: an approach to the isolation of regulatory mutants in Pseudomonas aeruginosa. J Bacteriol. 1972 Jan;109(1):365–372. doi: 10.1128/jb.109.1.365-372.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISUMI M. Studies on the isoleucine fermentation. I. Screening of organisms and investigation of cultural conditions. J Biochem. 1962 Dec;52:390–399. doi: 10.1093/oxfordjournals.jbchem.a127635. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Kato J., Komatsubara S., Chibata I. Increase in isoleucine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. Appl Microbiol. 1971 Apr;21(4):569–574. doi: 10.1128/am.21.4.569-574.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Multivalent repression and genetic depression of isoleucine-valine biosynthetic enzymes in Serratia marcescens. J Bacteriol. 1971 Sep;107(3):824–827. doi: 10.1128/jb.107.3.824-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Valine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. J Bacteriol. 1971 May;106(2):493–499. doi: 10.1128/jb.106.2.493-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine accumulation by regulatory mutants of Serratia marcescens: lack of both feedback inhibition and repression. J Bacteriol. 1972 May;110(2):761–763. doi: 10.1128/jb.110.2.761-763.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine hydroxamate, an isoleucine antagonist. J Bacteriol. 1971 Sep;107(3):741–745. doi: 10.1128/jb.107.3.741-745.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Properties of isoleucine hydroxamate-resistant mutants of Serratia marcescens. J Gen Microbiol. 1971 Dec;69(3):291–297. doi: 10.1099/00221287-69-3-291. [DOI] [PubMed] [Google Scholar]
- Kondo M., Woese C. R. Specificity of aminoacyl transfer ribonucleic acid synthetases from Escherichia coli K12. Biochemistry. 1969 Oct;8(10):4177–4182. doi: 10.1021/bi00838a040. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Silbert D. F., Fink G. R., Voll M. J., Antón D., Hartman P. E., Ames B. N. Transfer RNA and the control of the histidine operon. Cold Spring Harb Symp Quant Biol. 1966;31:383–392. doi: 10.1101/sqb.1966.031.01.050. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]


